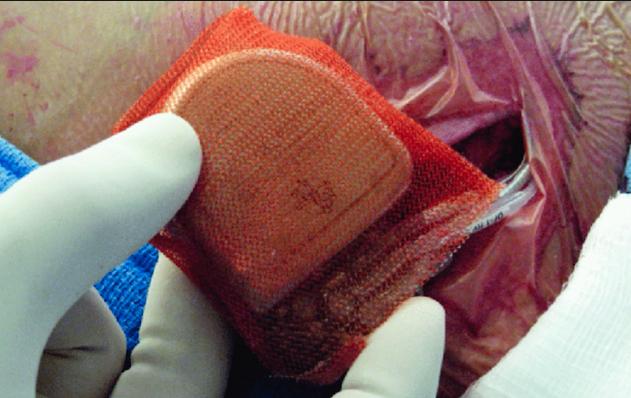
One of the cardiology technologies in the list of top advances in 2019 is Medtronic’s Tyrx Absorbable Antibacterial Envelope using to enclose cardiac implantable electronic devices (CIEDs). It reduced the risk of major infection by 40 percent, and pocket infection by 61 percent, based on data presented at ACC 2019.
November 6, 2019 — Cleveland Clinic announced the Top 10 Medical Innovations for 2020 at a multimedia presentation last month that capped off the 2019 Medical Innovation Summit. Now in its 17th year, the annual Medical Innovation Summit is organized by Cleveland Clinic Innovations, the development and commercialization arm of Cleveland Clinic.
The list of up-and-coming technologies was selected by a panel of Cleveland Clinic physicians and scientists, led by Michael Roizen, M.D., Emeritus Chief Wellness Officer at Cleveland Clinic.
“Healthcare is ever changing and we anticipate that these innovations will significantly transform the medical field and improve care for patients at Cleveland Clinic and throughout the world,” said Roizen.
Here, in order of anticipated importance, are the Top 10 Medical Innovations for 2020:
1. Dual-Acting Osteoporosis Drug
Osteoporosis is a condition in which bones become weak and brittle, effectively increasing their risk of breaking. With osteoporosis, the loss of bone occurs silently and progressively – often without symptoms until the first fracture. Providing more bone-strengthening power, the recent FDA approval of a new dual-acting drug (romosozumab) is giving patients with osteoporosis more control in preventing additional fractures.
2. Expanded Use of Minimally Invasive Mitral Valve Surgery
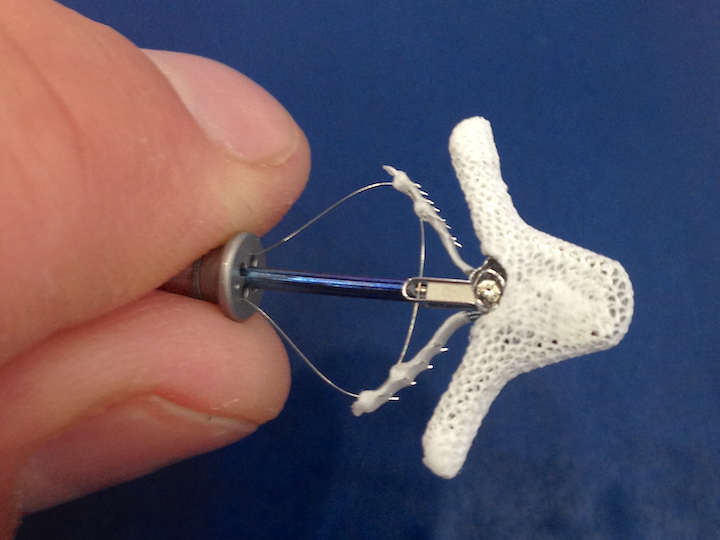 The mitral valve allows blood flow from the heart’s left atrium to the left ventricle. But in about 1 in 10 individuals over the age of 75, the mitral valve is defective causing the action of regurgitation. Expanding the approval of the Abbott MitraClip transcatheter mitral valve repair device to a population of patients who have failed to get symptom relief from other therapies provides an important new treatment option. In March 2019, the U.S. Food and Drug Administration (FDA) granted a new indication for the MitraClip to treat patients with heart failure symptoms and moderate-to-severe or severe secondary (functional) mitral regurgitation, despite optimal medical therapy. This was based on data from the landmark COAPT Trial, presented in late 2018. Read the article MitraClip Reduces Mortality for Heart Failure Patients With Secondary Mitral Regurgitation.
The mitral valve allows blood flow from the heart’s left atrium to the left ventricle. But in about 1 in 10 individuals over the age of 75, the mitral valve is defective causing the action of regurgitation. Expanding the approval of the Abbott MitraClip transcatheter mitral valve repair device to a population of patients who have failed to get symptom relief from other therapies provides an important new treatment option. In March 2019, the U.S. Food and Drug Administration (FDA) granted a new indication for the MitraClip to treat patients with heart failure symptoms and moderate-to-severe or severe secondary (functional) mitral regurgitation, despite optimal medical therapy. This was based on data from the landmark COAPT Trial, presented in late 2018. Read the article MitraClip Reduces Mortality for Heart Failure Patients With Secondary Mitral Regurgitation.
3. Inaugural Medication for Transthyretin Amyloid Cardiomyopathy
 A disheartening cardiovascular disorder, ATTR-CM is a progressive, underdiagnosed, potentially fatal disease in which amyloid protein fibrils deposit in, and stiffen, the walls of the heart’s left ventricle. But a new agent to prevent misfolding of the deposited protein is showing a significantly reduced risk of death. Following Fast-Track and Breakthrough designations in 2017 and 2018, 2019 marked the FDA approval of tafamidis, the first-ever medication for treatment of this increasingly recognized condition. With a treatment now available, there has been a renewed interest in cardiac nuclear imaging of amyloidosis.
A disheartening cardiovascular disorder, ATTR-CM is a progressive, underdiagnosed, potentially fatal disease in which amyloid protein fibrils deposit in, and stiffen, the walls of the heart’s left ventricle. But a new agent to prevent misfolding of the deposited protein is showing a significantly reduced risk of death. Following Fast-Track and Breakthrough designations in 2017 and 2018, 2019 marked the FDA approval of tafamidis, the first-ever medication for treatment of this increasingly recognized condition. With a treatment now available, there has been a renewed interest in cardiac nuclear imaging of amyloidosis.
4. Therapy for Mitigation of Peanut Allergies
It’s a terrifying reality for 2.5 percent of parents – the possibility that at any moment, their child might be unable to breathe due to an allergic reaction. Though emergency epinephrine has reduced the severity and risk of accidental exposure, these innovations are not enough to quell the ever-present anxiety. But development of a new oral immunotherapy medication to gradually build tolerance to peanut exposure holds the opportunity to lend protection against attack.
5. Closed-Loop Spinal Cord Stimulation
Chronic pain is a terribly frustrating condition, and a large reason for prescription of opioid medication. Spinal cord stimulation is a popular treatment for chronic pain through which an implantable device provides electrical stimulus to the spinal cord. But unsatisfactory outcomes due to subtherapeutic or overstimulation events are common. Closed-loop stimulation is allowing for better communication between the device and the spinal cord providing more optimal stimulation and relief of pain.
6. Biologics in Orthopaedic Repair
After orthopaedic surgery, the body can take anywhere from months to years to recover. But biologics – cells, blood components, growth factors and other natural substances – have the power to replace or harness the body’s own power and promote healing. These elements are finding their way into orthopaedic care, allowing for the possibility of expedited improved outcomes.
7. Antibiotic Envelope for Cardiac Implantable Device Infection Prevention
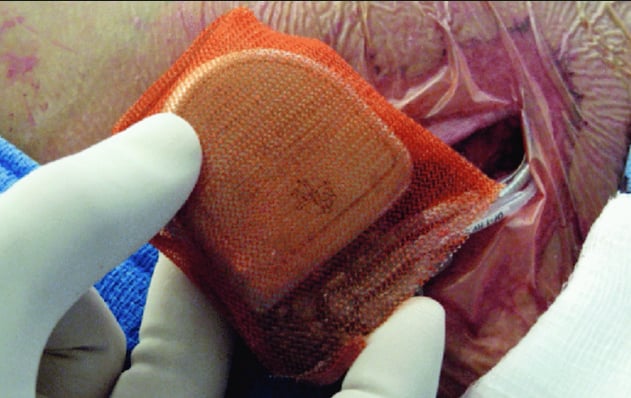 Worldwide, roughly 1.5 million patients receive an implantable cardiac electronic device every year. In these patients, infection remains a major, potentially life-threatening complication. Antibiotic-embedded envelopes are now made to encase these cardiac devices, effectively preventing infection. Results from the landmark Worldwide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT) were presented at the 2019 American College of Cardiology (ACC) meeting. Data demonstrated Medtronic’s Tyrx Absorbable Antibacterial Envelope reduced the risk of major infection by 40 percent, and pocket infection by 61 percent, in patients with cardiac implantable electronic devices (CIEDs). Watch the related VIDEO: Use of an Antibacterial Envelope to Reduce Infections for Pacemakers, ICDs — Interview with Khaldoun Tarakji, M.D., who presented the WRAP-IT Trial.
Worldwide, roughly 1.5 million patients receive an implantable cardiac electronic device every year. In these patients, infection remains a major, potentially life-threatening complication. Antibiotic-embedded envelopes are now made to encase these cardiac devices, effectively preventing infection. Results from the landmark Worldwide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT) were presented at the 2019 American College of Cardiology (ACC) meeting. Data demonstrated Medtronic’s Tyrx Absorbable Antibacterial Envelope reduced the risk of major infection by 40 percent, and pocket infection by 61 percent, in patients with cardiac implantable electronic devices (CIEDs). Watch the related VIDEO: Use of an Antibacterial Envelope to Reduce Infections for Pacemakers, ICDs — Interview with Khaldoun Tarakji, M.D., who presented the WRAP-IT Trial.
8. Bempedoic Acid for Cholesterol Lowering in Statin Intolerant Patients
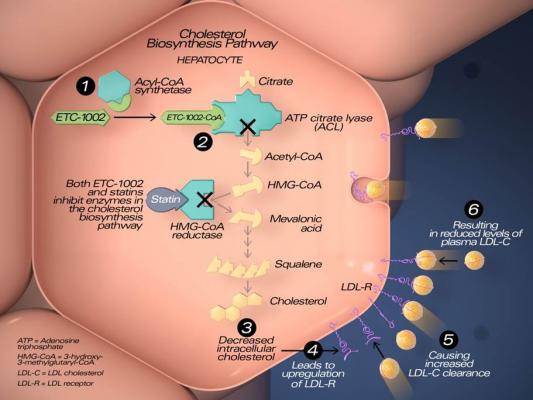 High cholesterol is a major concern for nearly 40 percent of adults in the U.S. Left untreated, the condition could lead to serious health problems like heart attack and stroke. Though typically managed with statins, some individuals experience unacceptable muscle pain with statins. Bempedoic acid provides an alternative approach to lowering of LDL-cholesterol while avoiding these side effects. Read the ACC 2019 late-breaking study article Bempedoic Acid Combination Tablet Significantly Lowers LDL-Cholesterol.
High cholesterol is a major concern for nearly 40 percent of adults in the U.S. Left untreated, the condition could lead to serious health problems like heart attack and stroke. Though typically managed with statins, some individuals experience unacceptable muscle pain with statins. Bempedoic acid provides an alternative approach to lowering of LDL-cholesterol while avoiding these side effects. Read the ACC 2019 late-breaking study article Bempedoic Acid Combination Tablet Significantly Lowers LDL-Cholesterol.
9. PARP Inhibitors for Maintenance Therapy in Ovarian Cancer
PARP, or poly-ADP ribose polymerase, inhibitors block repair of damaged DNA in tumor cells which increases cell death, especially in tumors with deficient repair mechanisms. One of the most recent important advances in ovarian cancer treatment, PARP inhibitors have improved progression-free survival and are now being approved for first-line maintenance therapy in advanced stage disease. Several additional large-scale trials are underway with PARP inhibitors set to make great strides in improving outcomes in cancer therapy.
10. Drugs for Heart Failure with Preserved Ejection Fraction
 Heart failure with preserved ejection fraction (HFpEF) – also known as diastolic heart failure – is the condition in which the ventricular heart muscles contract normally, but do not relax as they should. With preserved ejection fraction, the heart is unable to properly fill with blood – leaving less available to be pumped out to the body. Currently, recommendations for this treatment are directed at accompanying conditions and mere symptom relief. But SGLT2 inhibitors, a class of medications used in the treatment of type 2 diabetes, is now being explored in HFpEF – alluding to a potential new treatment option. In October 2019, the U.S. Food and Drug Administration (FDA) cleared AstraZeneca's dapagliflozin (Farxiga) to reduce the heart failure hospitalizations in adults with type 2 diabetes (T2D) and established cardiovascular disease (CVD) or multiple cardiovascular (CV) risk factors. Read the ACC 2019 late-breaking study article Diabetes Drug Effective Against Heart Failure in Wide Spectrum of Patients.
Heart failure with preserved ejection fraction (HFpEF) – also known as diastolic heart failure – is the condition in which the ventricular heart muscles contract normally, but do not relax as they should. With preserved ejection fraction, the heart is unable to properly fill with blood – leaving less available to be pumped out to the body. Currently, recommendations for this treatment are directed at accompanying conditions and mere symptom relief. But SGLT2 inhibitors, a class of medications used in the treatment of type 2 diabetes, is now being explored in HFpEF – alluding to a potential new treatment option. In October 2019, the U.S. Food and Drug Administration (FDA) cleared AstraZeneca's dapagliflozin (Farxiga) to reduce the heart failure hospitalizations in adults with type 2 diabetes (T2D) and established cardiovascular disease (CVD) or multiple cardiovascular (CV) risk factors. Read the ACC 2019 late-breaking study article Diabetes Drug Effective Against Heart Failure in Wide Spectrum of Patients.
For more information: www.innovations.clevelandclinic.org
Related Trends in Cardiovascular Technologies:
8 Cardiovascular Technologies to Watch in 2020 — Observations of DAIC Editor Dave Fornell
A 40,000 Foot View of Trends in Cardiology
New Technology Highlights on the ACC 2019 Exhibit Floor
6 Key Health Information Technology Trends at HIMSS 2019
Echocardiography Trends at ASE 2019
7 Hot Topics in Cardiac CT Imaging
Key Technology and News at AHA 2018
6 Hot Topics in Interventional Cardiology at TCT 2019
VIDEO: Editor's Choice of the Most Innovative New Cardiac Technology at AHA 2018


 November 14, 2025
November 14, 2025 









