August 4, 2022 — The Heart Rhythm Society (HRS) is a professional society representing a diverse population of ...
Pacemakers
This channel includes news and new technology innovations for pacemakers used to treat bradycardia.
June 9, 2022 — Holes help make sponges and English muffins useful (and, in the case of the latter, delicious). Without ...
May 17, 2022 — Heart Rhythm 2022 has come to a close, and the Heart Rhythm Society has released some stats regarding ...
Royal Philips, a global leader in health technology, announced late-breaking results of a large-scale, real-world ...
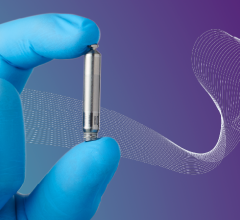
April 5, 2022 — Abbott announced that the U.S. Food and Drug Administration (FDA) has approved the Aveir single-chamber ...
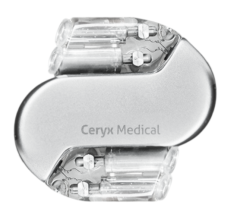
February 16, 2022 – A revolutionary pacemaker that re-establishes the heart’s naturally irregular beat is set to be ...
February 15, 2022 — UC Davis pediatric electrophysiologist Dan Cortez placed two objects onto the table in front of ...
February 14, 2022 — Philips, a global leader in health technology, is supporting the American Heart Association’s multi ...

Children’s Hospital Los Angeles cardiologist Michael Silka, M.D., helped to pioneer the development of indications for ...
Christine Albert, M.D., MPH, professor, chair of the Department of Cardiology and the Lee and Harold Kapelovitz ...
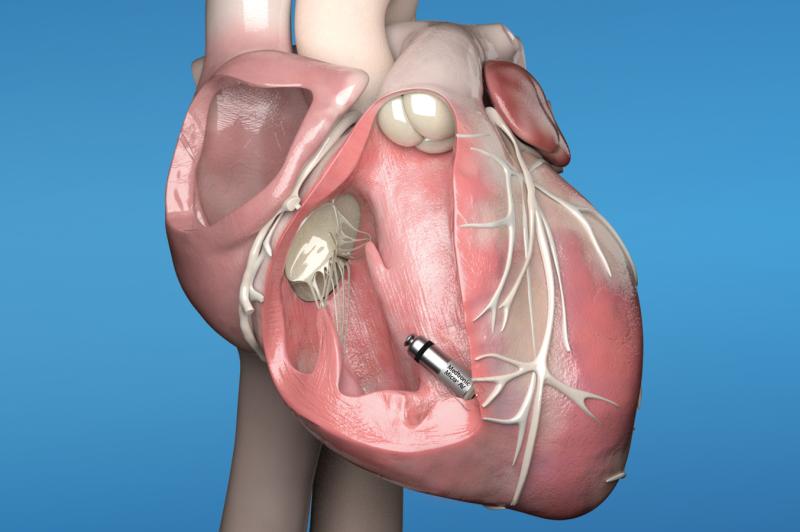
November 18, 2021 — The U.S. Food and Drug Administration (FDA) is reminding providers about the risk of major ...
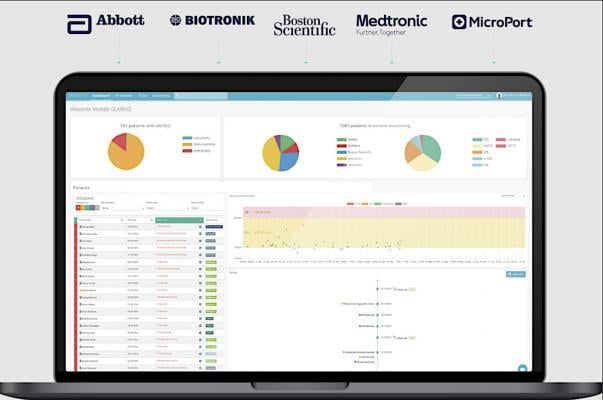
With 1.2 to 1.4 million new electrophysiology (EP) devices being prescribed to patients around the world each year ...
October 1, 2021 — The European Society of Cardiology (ESC) guidelines on cardiac pacing and cardiac resynchronization ...
Khaldoun Tarakji, M.D., MPH, associate section head, cardiac electrophysiology, Heart and Vascular Institute at ...
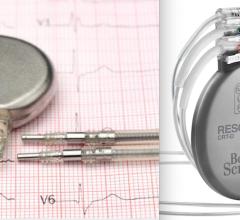
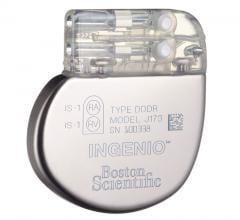
August 9, 2021 — Boston Scientific is recalling INGENIO family of pacemakers and CRT-Ps due to the risk of incorrect ...


 August 04, 2022
August 04, 2022