July 27, 2021 — Medtronic announced new data from the landmark WRAP-IT study published in Heart Rhythm,[1] demonstrating ...
Pacemakers
This channel includes news and new technology innovations for pacemakers used to treat bradycardia.

Cardiovascular diseases are among the leading causes of death for people over 65 years old in North America and Europe ...
June 2, 2021 — Implicity, a leader in remote patient monitoring software and cardiac data management solutions ...
May 19, 2021 — Among patients with both heart failure and atrial fibrillation (AFib), treatment strategies focused on ...
May 14, 2021 — The U.S. Food and Drug Administration (FDA) is advising patients and caregivers to keep any consumer ...
May 14, 2021 — Abbott is recalling a subset of Assurity and Endurity pacemakers built using specific manufacturing ...
April 19, 2021 — Former Vice President Mike Pence is recovering after having a pacemaker successfully implanted to ...
April 2, 2021 — Today, the American College of Cardiology (ACC) launched the Electrophysiology (EP) Device Implant ...

A few weeks ago, a patient of mine experienced a cardiac event — his heart stopped. Completely. However, his implantable ...
February 2, 2021 – Optimize EP, a digital health company focused on transforming cardiac care by improving the current ...
Robert Kowal, M.D., chief medical officer of the Medtronic cardiac rhythm and heart failure division, said there has ...
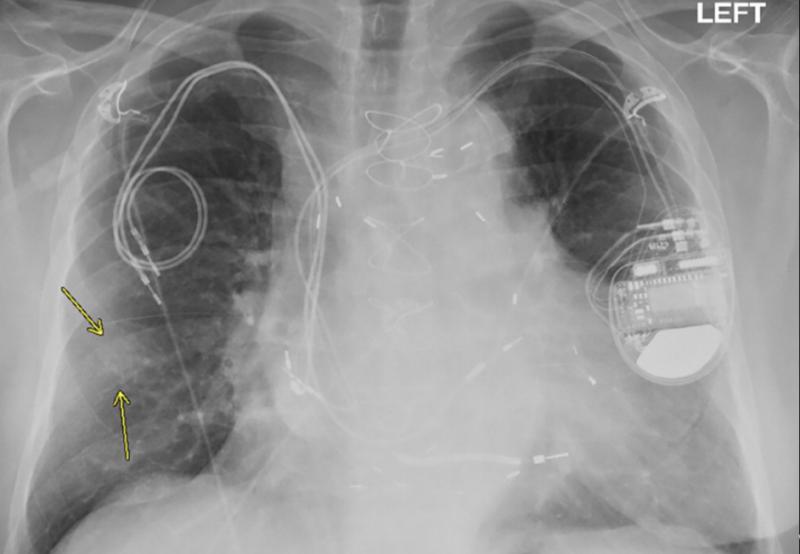
October 27, 2020 – Magnetic resonance imaging (MRI) examinations can be safely performed in patients with non-MR ...
June 16, 2020 - Biotronik has today announced its commitment to giving physicians additional tools to pace in the His ...
Interview with Andrew D. Krahn, M.D., FHRS, head of the division of cardiology at St. Paul’s Hospital, and professor of ...
Andrew Weintraub, M.D., FACC, associate director, of the Interventional Cardiology and Vascular Center, medical director ...

![Medtronic announced new data from the landmark WRAP-IT study published in Heart Rhythm,[1] demonstrating a significantly lower infection risk for patients who develop hematomas after cardiac implantable electronic devices (CIEDs) when the Tyrx Absorbable Antibacterial Envelope is used at implant. The analysis showed an 82% reduction in major CIED infections among patients with the Tyrx Envelope who developed hematomas compared to patients in the control group who developed hematomas.](/sites/default/files/styles/content_feed_medium/public/X0000_Tyrx_AIGISRx_Envelope_with_Pacemaker.jpg?itok=BHsWJ9_y)
 July 27, 2021
July 27, 2021