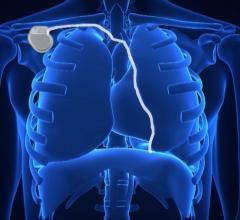
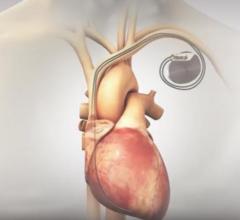
This video illustrates how the Micra AV leadless pacemaker is delivered via catheter and enables atrioventricular (AV) ...
Pacemakers
This channel includes news and new technology innovations for pacemakers used to treat bradycardia.
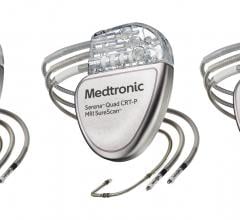
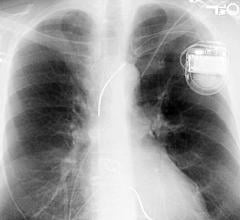
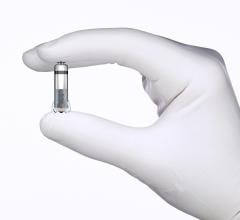
February 13, 2020 — The U.S. Food and Drug Administration (FDA) has approval of Micra AV, the world’s smallest pacemaker ...
(February 2020 update — the results of this trial led to the FDA clearnace of the Micra AV device in January 2020 - FDA ...
November 13, 2019 — Texas Heart Institute (THI) was awarded a prestigious four-year, $2.39 million grant from the Nation ...
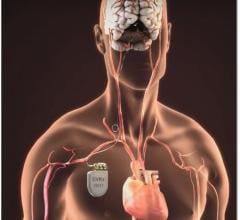
October 10. 2019 — Late-breaking results from its MODERATO II double-blind, randomized study of BackBeat Cardiac ...
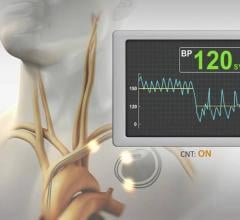
August 19, 2019 — The U.S. Food and Drug Administration (FDA) granted market clearance the Barostim Neo System for the ...
July 31, 2019 — The chances of patients experiencing complications after having a cardiac device implanted vary ...
June 4, 2019 — Orchestra BioMed Inc. announced the presentation of two-year clinical data from the European Moderato I ...

May 15, 2019 — The Heart Rhythm Society (HRS) had 21 late-breaking study presentations at the 2019 Heart Rhythm ...
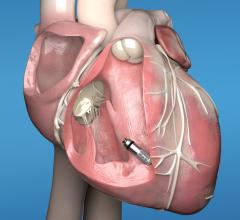
May 15, 2019 — A new infection risk scoring system has been developed based on data from the large PADIT Trial.[1] The ...
May 14, 2019 – Results from new research show that passengers with cardiac implantable electronic devices (CIEDs), such ...
May 13, 2019 – Results from a new survey are the first to report a large discrepancy in patient’s knowledge of their ...
May 7, 2019 — The U.S. Food and Drug Administration (FDA) issued a safety communication to alert healthcare providers ...
March 29, 2019 — A research team from Imperial College London believes a new software could speed up the diagnosis and ...
Khaldoun Tarakji, M.D., MPH, associate section head, section of electrophysiology and pacing in the Robert and Suzanne ...


 February 13, 2020
February 13, 2020

![A new infection risk scoring system has been developed based on data from the large PADIT Trial.[1] The new scoring system was presented as a follow up to that study during a late-breaking session at Heart Rhythm 2019, the Heart Rhythm Society's 40th Annual Scientific Sessions.](/sites/default/files/styles/content_feed_medium/public/PADIT_Infection_Risk_score.jpg?itok=O1-YAcMm)