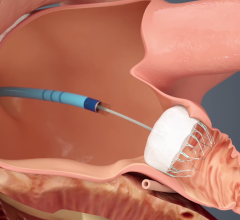
November 7, 2016 – Results from the U.S. real-world, post-FDA approval experience of the Watchman device found high ...
Structural Heart Occluders
This channel includes news and new technology innovations about structural heart occluders. These include information of surgical repair and transcatheter closure of PFO, VSD, ASD and LAA. Devices include the Amplatzer and the Gore Helex

November 2, 2016 — St. Jude Medical Inc. announced the long-term data from RESPECT, a landmark trial, during a First ...
November 2, 2016 — St. Jude Medical Inc. announced this week the U.S. Food and Drug Administration (FDA) approval and ...
October 19, 2016 — St. Jude Medical Inc. announced the launch of the ADO II AS (AMPLATZER Duct Occluder II Additional ...
September 2, 2016 — St. Jude Medical Inc. announced the start of the St. Jude Medical Amplatzer Amulet IDE trial of the ...
August 8, 2016 — Updated recommendations from the American Academy of Neurology (AAN) states that catheter-based closure ...
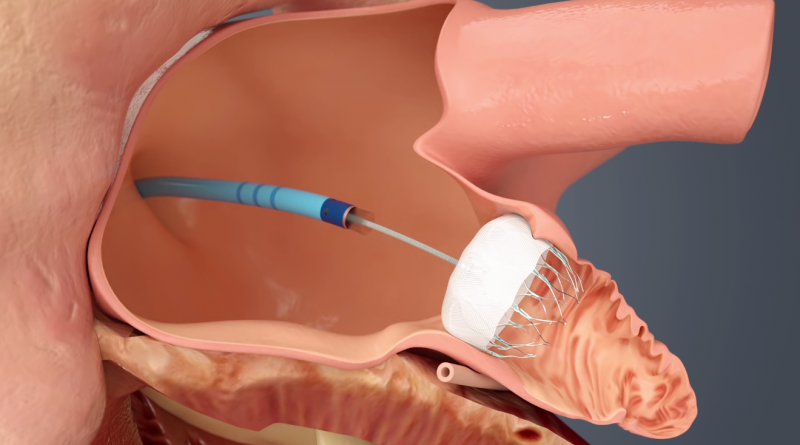
Patients with atrial fibrillation (AF or Afib) are high risk for stroke due to the formation of thrombus emboli in the ...
This is an animation, supplied by Gore, demonstrated how the Cardioform Septal Occluder is implanted for the ...
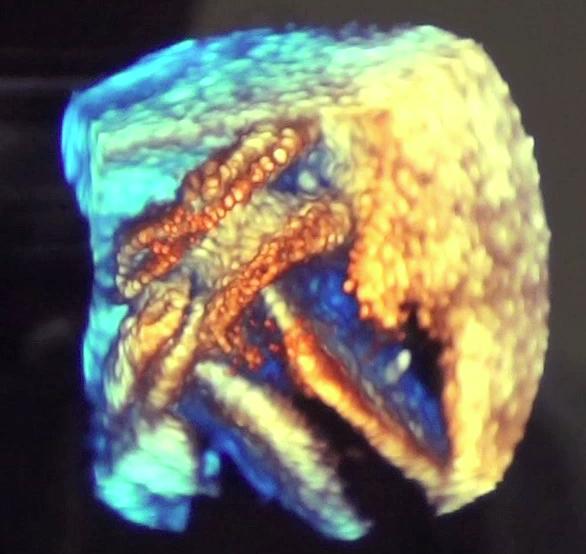
May 27, 2016 — A U.S. Food and Drug Administration (FDA) panel recommended approval of a transcatheter patent foramen ...

Atrial fibrillation (AF) affects nearly 6 million Americans and the condition puts them at significantly greater risk of ...
David Holmes, M.D., professor of medicine, Mayo Clinic College of Medicine and consultant, Department of Internal ...
February 9, 2016 — Boston Scientific Corp. announced the Centers for Medicare and Medicaid Services (CMS) will cover ...
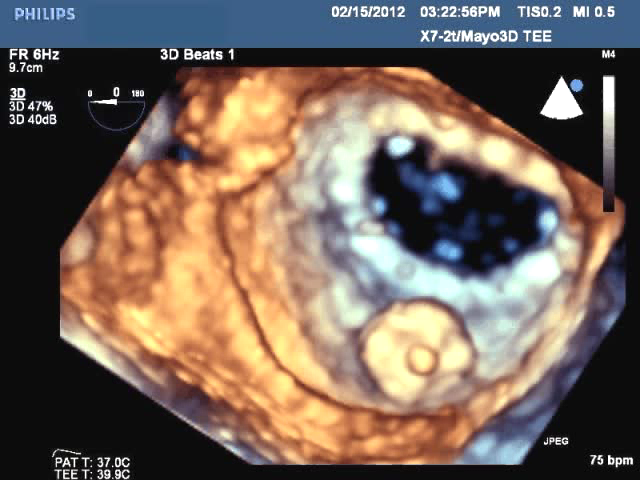
Off-label use of the St. Jude Amplatzer vascular plug devices offers a new solution for the minimally invasive repair of ...
January 20, 2016 — On the 100th anniversary of the Endurance expedition to Antarctica led by Sir Ernest Shackleton ...
DAIC Editor Dave Fornell offers his choices for the most innovative new interventional cardiovascular technologies ...

 November 07, 2016
November 07, 2016