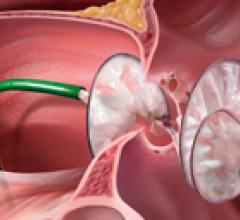
October 4, 2010 – Germany will reimburse patients who have a left atrial appendage closure procedure in 2011. The ...
Structural Heart Occluders
This channel includes news and new technology innovations about structural heart occluders. These include information of surgical repair and transcatheter closure of PFO, VSD, ASD and LAA. Devices include the Amplatzer and the Gore Helex
September 28, 2010 – The Transcatheter Cardiovascular Therapeutics (TCT) 2010 symposium featured a live case ...
September 23, 2010 – The first consensus document for treating structural heart disease has been published by the ...

Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is a major risk factor for developing ...
August 25, 2010 – Alleging infringement of a patent for its transcatheter cardiac septal occluder devices, AGA ...
August 25, 2010 – There will be special tracks available for nurses and technologists and for the transcatheter ...
August 12, 2010 – A 52-year-old male heart failure patient in Krakow, Poland, became the first human to receive ...
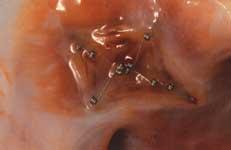
In addition to the FDA-cleared Gore Helex and the AGA Amplatzer transcatheter devices for atrial septal defects ...

(This article was updated with new information in June 2016) Transcatheter structural heart repair devices ...
July 9, 2010 – W. L. Gore and Associates announced today that they are confident in the design and anticipated ...
June 28, 2010 – AGA Medical has been denied the right to appeal a decision in a United Kingdom patent case ...
June 17, 2010 – AGA Medical Holdings Inc. this week announced first patient enrollment in the feasibility phase of ...
June 16, 2010 – A new surgical device for excluding the left atrial appendage has received clearance from the U.S ...
March 29, 2010 – A lawsuit was settled between Medtronic and AGA Medical Corp. for the alleged infringement of ...
March 25, 2010 – The FDA this week granted permission for a conditional investigational device exemption (IDE) to ...


 October 04, 2010
October 04, 2010