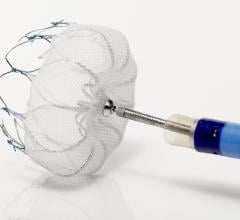
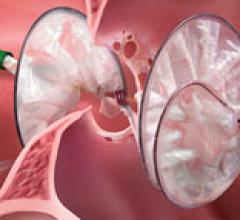
October 16, 2015 — Long-term study results from the RESPECT trial found that closing a patent foramen ovale (PFO) with ...
Structural Heart Occluders
This channel includes news and new technology innovations about structural heart occluders. These include information of surgical repair and transcatheter closure of PFO, VSD, ASD and LAA. Devices include the Amplatzer and the Gore Helex
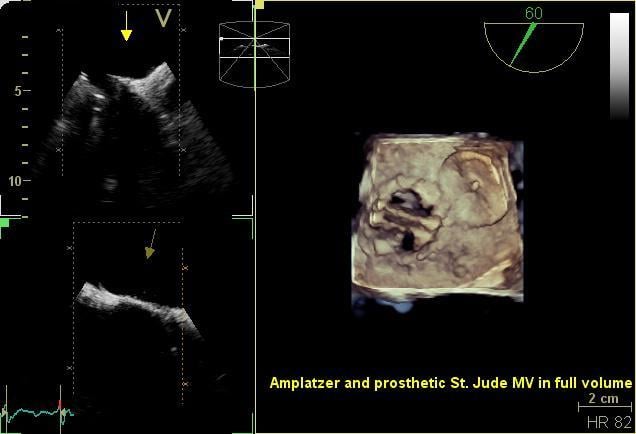
August 26, 2014 — The Cardiovascular Research Foundation (CRF) announced the late-breaking trials and first report ...
Interview with Steve Goldstein, M.D., director, noninvasive lab, Medstar Washington Heart Institute, Washington, D.C ...
Role of Interventional Echcardiography in Transcatheter Structural Heart Procedures — Rebecca Hahn, M.D., Columbia ...
Interview with Ted Feldman, M.D., FACC, MSCAI, FESC, cardiac cath lab director, Evanston Hospital, North Shore Health ...
Interview with Charanjit Rihal, M.D., chief of cardiology and professor of medicine, Mayo Clinic, at the Transcatheter ...
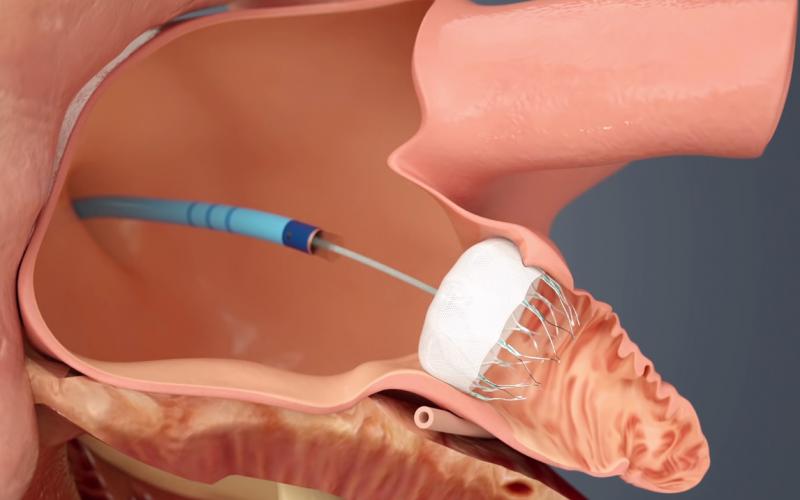
The long-awaited U.S. Food and Drug Administration (FDA) approval of the first transcatheter left atrial appendage (LAA) ...
March 16, 2015 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) approval for the Watchman ...
October 13, 2014 — After reviewing updated data and analysis for the Boston Scientific Watchman left atrial appendage ...
August 1, 2014 — Occlutech announced that it has successfully obtained a ruling by the U.K. Patents Court in the High ...


August 30, 2013 — PFM Medical received U.S. Food and Drug Administration (FDA) premarket approval (PMA) for its Nit ...
August 7, 2013 — W. L. Gore & Associates announced a favorable ruling involving the Gore Helex Septal Occluder. The Hon ...

 October 16, 2015
October 16, 2015