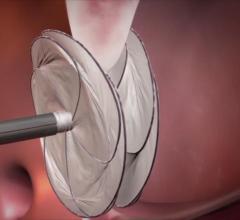
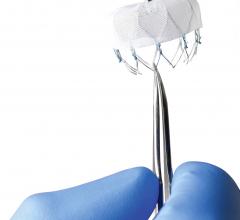
April 3, 2018 — Following the unprecedented Gore REDUCE Clinical Study conclusion that closure of patent foramen ovale ...
Structural Heart Occluders
This channel includes news and new technology innovations about structural heart occluders. These include information of surgical repair and transcatheter closure of PFO, VSD, ASD and LAA. Devices include the Amplatzer and the Gore Helex

There were several notable presentations of new data on cardiovascular technologies at the recent 2018 American College ...
March 20, 2018 — Among people with a type of hole in the heart, known as patent foramen ovale (PFO), those who received ...
Vivek Reddy, M.D., director of cardiac arrhythmia services and professor of medicine, cardiology, Mount Sinai Hospital ...
John Rhodes, M.D., co-director of the adult congenital heart program, Medical University of South Carolina, is the ...

November 2, 2017 – Five-year results from the PREVAIL Trial comparing left atrial appendage closure (LAAC) with the ...
Ziyad Hijazi, M.D., MPH, MSCAI, FACC, director of the cardiac program and chair of the Department of Pediatrics at Sidra ...
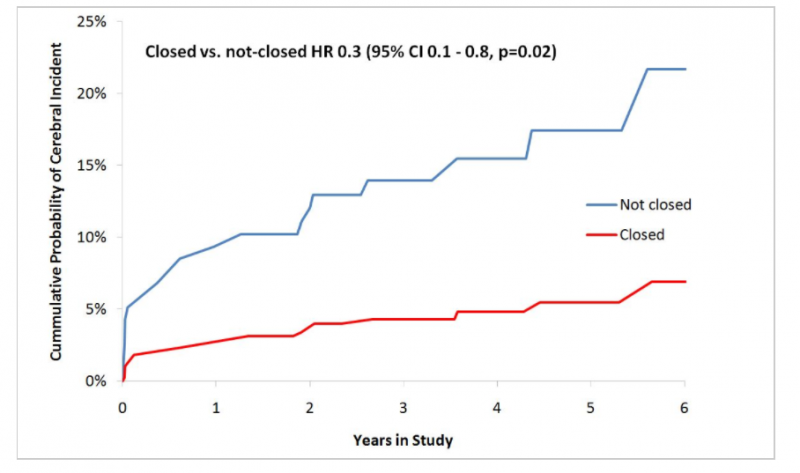
September 7, 2017 — Closure of the left atrial appendage (LAA) during heart surgery protects the brain, according to ...
September 5, 2017 — Abbott announced it has initiated a U.S. pivotal clinical study evaluating the safety and ...
May 30, 2017 — Data was positive for safety and efficacy rates of the Watchman Left Atrial Appendage Closure (LAAC) ...

March 29, 2017 — For patients with atrial fibrillation (AFib), closing the area of the heart known as the left atrial ...

March 3, 2017 — A Henry Ford Hospital study found a 100 percent success rate in left atrial appendage (LAA) occlusion ...
February 15, 2017 — Doctors at the University of Alabama at Birmingham have implemented the first U.S. Food and Drug ...
February 1, 2017 — Baylor Heart and Vascular Services at Fort Worth in November became the first program in Texas to ...
January 23, 2017 — Over 93 percent of heart attack patients are receiving stents within the guideline-recommended ...

 April 03, 2018
April 03, 2018