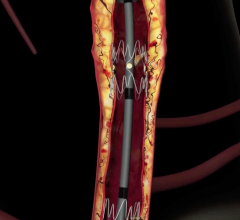
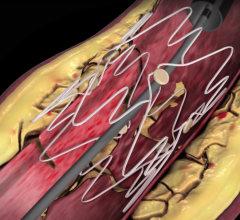
December 22, 2008 - C. R. Bard Inc. recently received FDA clearance to market the E-Luminexx Vascular Stent - a flexible, self-expanding nitinol stent.
The E-Luminexx is intended to treat patients with common or external iliac artery occlusive disease. Current estimates indicate each year more than 140,000 patients are candidates for iliac stent procedures in the U.S. This patient population is expected to grow by more than 10 percent annually due to the increased clinician focus on treating peripheral arterial disease and the growing number of diabetic patients who are at higher risk of developing these occlusions.
Bard’s prospective, multi-center, non-randomized, clinical study of 134 patients measuring the E-Luminexx Vascular Stent against objective performance criteria demonstrated a nine-month primary patency of 94 percent and a site reported anatomic success rate (
For more information: www.crbard.com


 November 06, 2019
November 06, 2019