June 12, 2008 - Sorin Group closed the sale of its peripheral stent business to Datascope Corp., divesting its noncore assets in order to better focus on its strategic businesses, cardiopulmonary, heart valves and CRM.
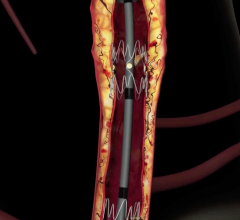
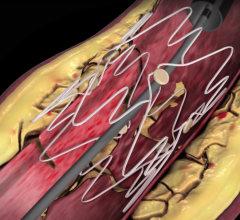
Sorin's peripheral stent products are dedicated to the treatment of peripheral arterial disease ("PAD") and use the Carbofilm Technology, whose excellent biocompatible and hemocompatible characteristics are clinically well proven.
Datascope has also received exclusive, worldwide rights within the endovascular field of use to the cCarbofilm technology as part of the deal. The product line includes balloon-expandable and self-expanding stent systems principally for use in the treatment of the iliac arteries, renal arteries and infrapopliteal lesions, as well as balloon systems for use in PTA (percutaneous transluminal angioplasty).
For more information: www.datascope.com


 November 06, 2019
November 06, 2019