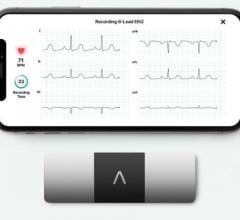
November 18, 2021 — A novel software algorithm compatible with a wide range of smartwatches and fitness trackers ...
November 18, 2021 — The U.S. Food and Drug Administration (FDA) is reminding providers about the risk of major ...
Dr. Simon Dixon, MBChB, chair of the Department of Cardiovascular Medicine at Beaumont Hospital Royal Oak, the Dorothy ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
November 17, 2021 — Taking daily low-dose aspirin for seven years did not affect the risk of dementia or mental decline ...
November 17, 2021 — People with atrial fibrillation (AFib) who underwent individualized testing to discover triggers for ...
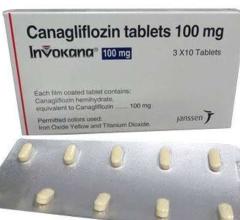
November 17, 2021 — Canagliflozin, a medication used to treat type 2 diabetes, was found to greatly improve symptoms and ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
November 17, 2021 — People in the U.S. who live in socially vulnerable neighborhoods, which includes communities with ...
November 17, 2021 — For people experiencing severe aortic stenosis who do not have symptoms or need symptom relief ...
November 16, 2021 — Acutus Medical Inc. (Acutus), an arrhythmia management company focused on improving the way cardiac ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Srihari Naidu, M.D., FACC, FAHA, FSCAI, is the director of both the cardiac cath labs and Hypertrophic Cardiomyopathy ...
November 15, 2021 — Esperion announced the presentation of three abstracts highlighting Nexletol tablets at the American ...
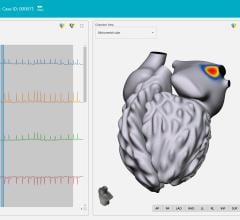
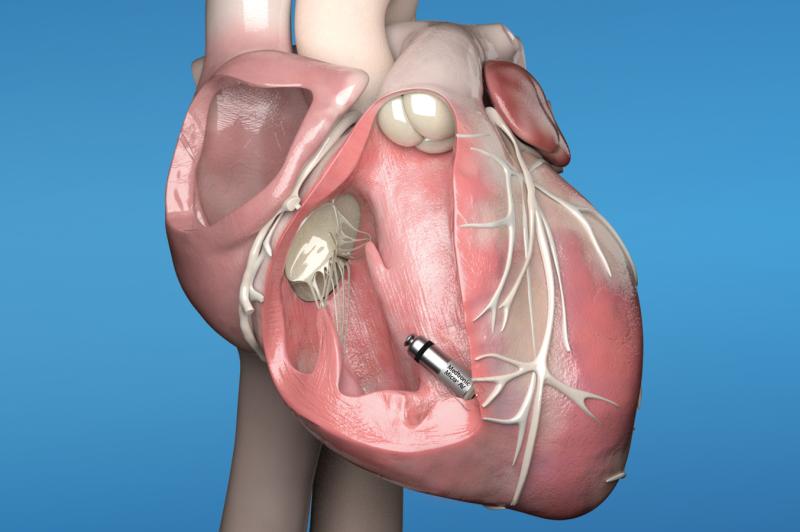
November 15, 2021 — Vektor Medical Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance for its ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...

November 15, 2021 — RealView Imaging Ltd. recently received FDA 510(k) clearance for its Holoscope-i holographic system ...
November 15, 2021 — Novartis announced results from a pooled post-hoc analysis of Phase III ORION-9, -10 and -11 trials ...
November 12, 2021 — Penumbra Inc. announced that the CHEETAH clinical study of its Indigo System CAT RX Catheter ...


 November 18, 2021
November 18, 2021