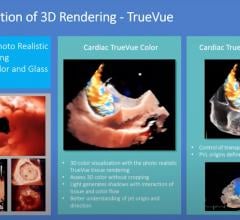
This on-demand webinar teaches viewers how new multi-planar imaging techniques such as Cardiac MultiVue and TrueVue make ...
April 26, 2021 – Cardiovascular diseases are the leading cause of death in the United States. Heart failure ...
The resounding success of transcatheter aortic valve replacement (TAVR) has led the creation of hundreds of structural ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 21, 2021 — EnsiteVascular announced it received its second U.S. Food and Drug Administration (FDA) market ...
The adoption of structured reporting within cardiology imaging departments is on the rise. Providers recognize the added ...
As a medical technology journalist, a little more than a decade ago I found myself sitting in those late afternoon ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 20, 2021 – The Minneapolis Heart Institute Foundation (MHIF) announced the first publication of outcomes from the ...
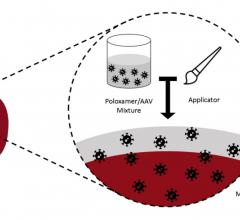
April 20, 2021 — Start-up medical device company atHeart Medical announced it is initiating its U.S. investigational ...
April 19, 2021 — Former Vice President Mike Pence is recovering after having a pacemaker successfully implanted to ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
April 19, 2021 — National Heart, Lung, and Blood Institute (NHLBI) has awarded a grant for $462,689 to Rhythm ...
April, 14, 2021 – Elixir Medical recently announced the first patient was treated in the BIOADAPTOR randomized ...
April 14, 2021 — The U.S. Food and Drug Administration (FDA) cleared the Acutus Medical AcQCross family of universal ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
(This story was updated May 7, 2021 in the last subheaded section) April 13, 2021 — The U.S. Food and Drug ...
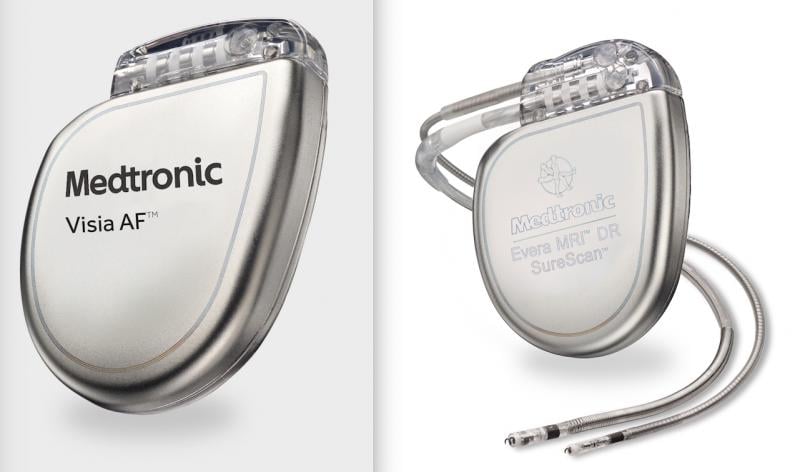
April 12, 2021 — Medtronic is recalling some of its implantable cardioverter defibrillators (ICD) and cardiac ...
April 8, 2021 — Abbott today announced it has received European CE mark and Health Canada authorization this week for ...

 April 26, 2021
April 26, 2021