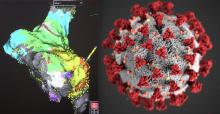
In our most challenging and distressing days, it is nice to think about a post-COVID-19 world.
But while this pandemic will end at some point, it is quite unlikely that we will completely eradicate the novel coronavirus that causes COVID-19 (SARS-CoV-2) — the way we did, for instance, with the virus that causes smallpox.