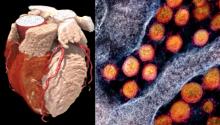
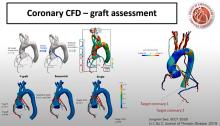
COVID-19 is creating a new generation of cardiac patients. With heart disease already the leading cause of deaths globally, this epidemic within a pandemic — a crisis within a crisis — creates a cardiology conundrum.
In all my years involved in healthcare, this is the most massive medical mystery I’ve encountered. The way patients are triaged, diagnosed, treated and monitored will forever change because of COVID-19. Perhaps nowhere has that been more evident than in the field of cardiology.