Feb. 3, 2026 — The first official summit of the European Association of Percutaneous Cardiovascular Interventions (EAPCI ...
Feb. 3, 2026 — Medtronic plc announced it will exercise its option to acquire CathWorks, a privately held medical device ...
Feb. 3, 2026 — Bristol Myers Squibb has launched "Change the Target. Change What’s Possible," an educational campaign ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Feb. 2, 2026 — Brainomix, a provider of AI-powered imaging tools in stroke and lung fibrosis, is introducing its new ...
Jan. 31, 2026 — The Society of Thoracic Surgeons (STS) has elected Vinay Badhwar, MD, as its 62nd president during the ...
Feb. 2, 2026 — GE HealthCare has announced that Allia Moveo has received U.S. Food and Drug Administration (FDA) 510(k) ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Jan. 28, 2026 — Corify Care has announced a major development in cardiac electrophysiology with the publication of its ...
Jan. 28, 2026 — Imperative Care has announced the commercial launch and first patient cases of the new Zoom 4S Catheter ...
Jan. 27. 2026 — Circle Cardiovascular Imaging Inc. has announced the release of cvi42 v6.4, the latest version of its ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
Jan. 27, 2026 — BURL Concepts, a private company developing a rapid, automated and affordable test for evaluating ...
Jan. 27, 2026 — Cytokinetics, Inc. has announced that Myqorzo (aficamten) is now available for prescription in 5 mg, 10 ...
Jan. 27, 2026 — A new national study reveals a stark disconnect between Americans’ desire for preventive cardiac ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
Jan. 27, 2026 — Robocath has launched the world’s first FIH (First-In-Human) clinical study evaluating its new robotic ...
Jan. 26, 2026 — AISAP, a provider of AI point-of-care diagnostics, has published a new clinical study in the peer ...
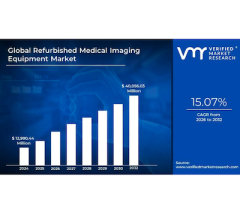
Jan. 11, 2026 — The Global Refurbished Medical Imaging Equipment Market Size is projected to grow at a CAGR of 15.07% ...


 February 04, 2026
February 04, 2026