University of Southampton scientists have discovered a link between coronary heart disease and osteoporosis, suggesting both conditions could have similar causes.

Boston Scientific has initiated a worldwide study to evaluate the rate and causes of shocks for patients implanted with the Emblem subcutaneous implantable defibrillator (S-ICD). The device is indicated for primary prevention of sudden cardiac death in the setting of severely reduced cardiac function (left ventricular ejection fraction </= 35 percent).
Medtronic plc announced it has acquired the assets of Aptus Endosystems Inc., a privately held medical device company focused on developing advanced technology for endovascular aneurysm repair (EVAR) and thoracic endovascular aneurysm repair (TEVAR). Medtronic completed its acquisition of the assets of Aptus Endosystems in a transaction valued at approximately $110 million. Additional terms of the acquisition were not disclosed.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
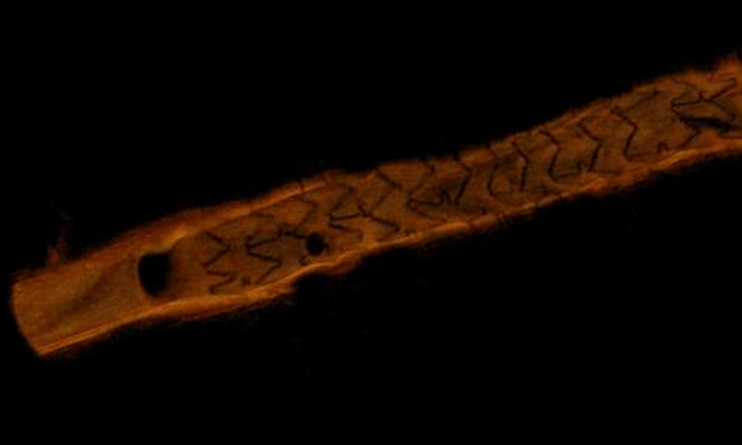
Angiography alone for procedural image guidance in the catheterization lab is sometimes not enough, and it is helpful to augment X-ray lumen imaging with intravascular ultrasound (IVUS) or optical coherence tomography (OCT) to visualize the soft tissue morphology.
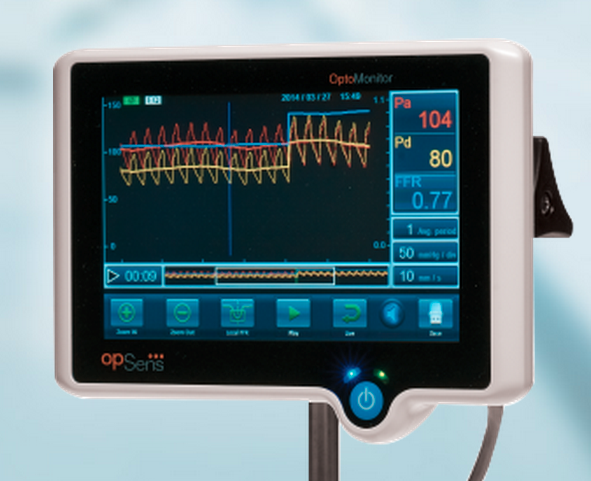
Key to ongoing U.S. healthcare reform are efforts to reduce costs by eliminating unnecessary medical procedures and using information technology (IT) to justify costs that are incurred to make providers more accountable. Key medical societies in each subspecialty will be responsible for what standards of care should be set and what guidelines should be followed. In the cath lab, fractional flow reserve (FFR) is already viewed as the gold standard for determining the hemodynamic impact of coronary lesions, acting as a gatekeeper to whether a patient is stented or receives medical therapy alone. For this reason, FFR is already being predicted as the measurement of record that will be required in documentation for stent reimbursement in the future.
In recent years, there has been widespread media coverage of studies purporting to show that radiation from X-rays, computed tomography (CT) scans and other medical imaging causes cancer. But such studies have serious flaws, including their reliance on an unproven statistical model, according to a recent article in the journal Technology in Cancer Research & Treatment. Corresponding author is Loyola University Medical Center radiation oncologist James Welsh, M.D., MS.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The American College of Cardiology (ACC) Statin Intolerance App is now available to help guide clinicians through the process of managing and treating patients who report muscle symptoms while on statin therapy.

Medtronic has acquired CardioInsight Technologies Inc., a Cleveland-based medical device company that has developed a new approach to improve the mapping of electrical disorders of the heart. CardioInsight will become part of the Medtronic atrial fibrillation solutions business in the cardiac rhythm and heart failure division.
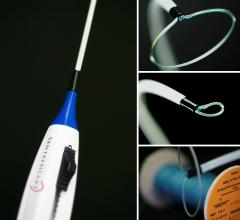
SentreHeart Inc. announced that it has received approval for an Investigational Device Exemption (IDE) from the U.S. Food and Drug Administration (FDA) to begin enrollment in a clinical study of the Lariat Suture Delivery Device. The randomized, controlled clinical study, known as the AMAZE Trial, will evaluate the use of the Lariat device for the ligation, or closure, of the left atrial appendage (LAA) as an adjunctive treatment to ablation in patients with persistent or longstanding persistent atrial fibrillation (AFib).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

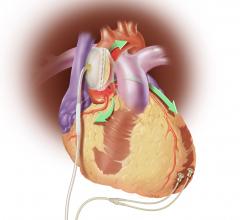
CardiAQ Valve Technologies (CardiAQ) announced that its second-generation transcatheter bioprosthetic mitral heart valve was successfully implanted as a compassionate treatment. The device was placed into a 72-year-old male suffering from severe mitral regurgitation (MR 4+) with multiple co-morbidities and ineligible for alternate treatment modalities.
Cardiac Dimensions announced the first patients have been enrolled in the REDUCE FMR clinical trial. REDUCE FMR is a prospective, double-blind, randomized multi-center trial, evaluating the company’s minimally-invasive Carillon Mitral Contour System.

The American College of Cardiology (ACC), Heart Rhythm Society (HRS) and Society for Cardiovascular Angiography and Interventions (SCAI) released a new overview on the implantation of left atrial appendage occlusion devices.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Merge Healthcare Inc. announced that it has entered into a strategic partnership with Toshiba America Medical Systems, Inc. Under this new agreement, Toshiba will be able to offer its customers a full turnkey solution, combining Merge's cardiology picture archiving and communication system (PACS) and hemodynamic monitoring solutions with the Toshiba portfolio of vascular X-ray systems.
Sunshine Heart Inc. has borrowed an additional $2 million to continue work on its U.S. pivotal study for the C-Pulse Heart Assist System. The minimally invasive surgically implanted device is designed to reduce and potentially reverse heart failure symptoms.
Cybersecurity was identified as an increased business priority over the past year according to 87 percent of respondents in the newly released 2015 HIMSS (Healthcare Information and Management Systems Society) Cybersecurity Survey. Two-thirds of those surveyed also indicated that their organizations had experienced a significant security incident recently. Released at the Privacy and Security Forum, held in Chicago from June 30-July 1, this research reflects the continued cybersecurity concerns by healthcare providers regarding the protection of their organizations' data assets.


 July 02, 2015
July 02, 2015