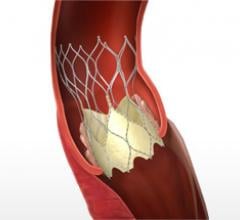
According to a new study, administering the blood thinner bivalirudin to patients experiencing an ST-elevation myocardial infarction (STEMI) in a pre-hospital setting can reduce the risk of death and major bleeding complications compared to heparin with optional use of glycoprotein IIb/IIIa inhibitors.
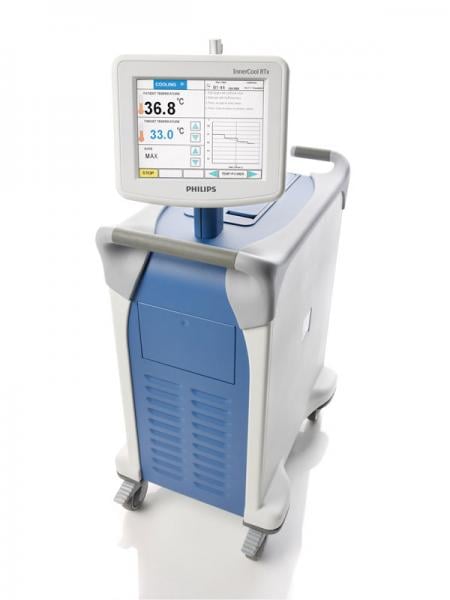
A clinical trial shows that rapidly cooling patients who have suffered ST-elevation myocardial infarction (STEMI) prior to restoring blood flow is safe and feasible. The findings of the CHILL-MI trial were presented at the 25th annual Transcatheter Cardiovascular Therapeutics scientific symposium (TCT 2013).

A study supports the use of instantaneous wave-free ratio (iFR) to simplify assessment and determine the severity of coronary artery disease (CAD). ADVISE II findings were presented today at the 25th annual Transcatheter Cardiovascular Therapeutics scientific symposium (TCT 2013).
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
A study found that both drug-eluting stents (DES) with biocompatible polymers and DES with biodegradable polymers were associated with low major adverse coronary events, demonstrating the non-inferiority of the biocompatible polymer stents in patients undergoing percutaneous coronary intervention (PCI).

A clinical trial conducted exclusively in women suggests that an initial strategy of using the radial artery in the arm as the entry point for cardiac catheterization or percutaneous coronary intervention (PCI) in women has potential for reducing bleeding complications.
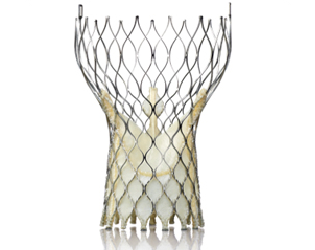
In a clinical trial, a self-expanding transcatheter aortic valve met the key performance objective of reducing death and stroke in patients with severe aortic stenosis at “extreme risk” for surgery. Results of the CoreValve Extreme Risk trial were presented at the 25th annual Transcatheter Cardiovascular Therapeutics scientific symposium (TCT 2013) in San Francisco.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
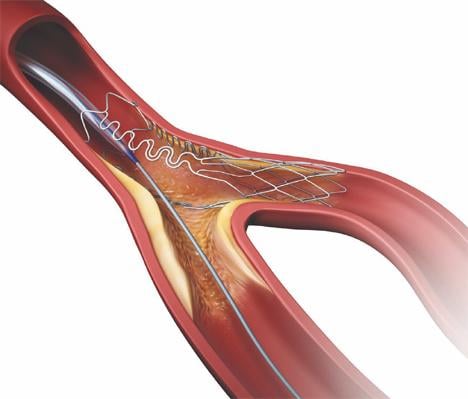
Tryton Medical Inc., a developer of stents designed to treat bifurcation lesions, announced activities highlighting the latest data and experience with the Tryton Side Branch Stent at the 25th annual Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium (TCT 2013).
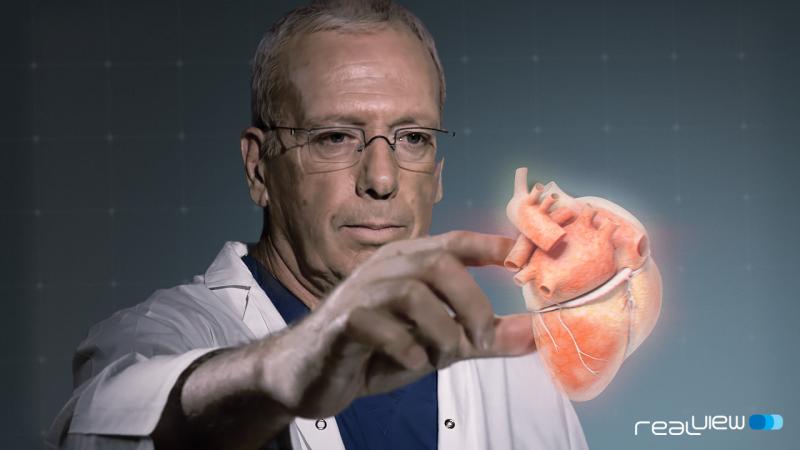
Philips and RealView Imaging Ltd. have completed a clinical study that demonstrated the feasibility of using an innovative live 3-D holographic visualization and interaction technology to guide minimally invasive structural heart disease procedures.

Boston Scientific Corp. has received CE mark for its Lotus Valve System, a transcatheter aortic valve replacement (TAVR) technology. This key approval offers an effective new treatment alternative for patients with severe aortic stenosis at high risk with surgical valve replacement.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
St. Jude Medical Inc. announced positive results for the 23 and 25mm Portico Transcatheter Aortic Heart Valves in the Portico Transfemoral CE mark trial (Portico TF CE Trial). Patients enrolled in the study experienced a significant improvement in valve function at 30 days.
Patients treated with the Boston Scientific Vessix Renal Denervation System experienced a significant and sustained reduction in blood pressure, according to new data presented today at the Transcatheter Cardiovascular Therapeutics Conference (TCT 2013) in San Francisco. An interim analysis of 139 patients enrolled in the REDUCE-HTN post market study affirms the device safety profile and effective treatment for resistant hypertension.
Abbott announced it plans to initiate a randomized, controlled trial in the United States to evaluate the use of dual anti-platelet therapy (DAPT) for a three-month duration following treatment with the company's Xience family of drug eluting stents (DES).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Amaranth Medical, a privately held medical device company, presented positive six-month angiographic results from its first-in-human study including patients undergoing percutaneous coronary intervention (PCI) with single coronary lesions.
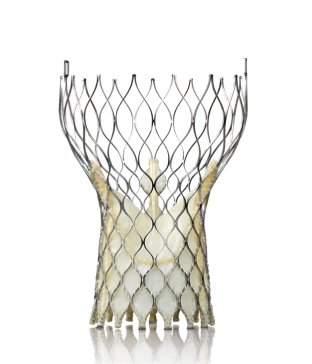
The CoreValve U.S. pivotal trial showed very positive results for the Medtronic CoreValve system, comparable or better than data from the Sapien Partner Trial.
Terumo Interventional Systems announced the launch of the 0.018-inch Glidewire Advantage peripheral guidewire during the 25th annual Transcatheter Cardiovascular Therapeutics (TCT) conference in San Francisco.

 November 01, 2013
November 01, 2013