Royal Philips Electronics showcased its latest addition to the ClearVue ultrasound family of products at the American College of Obstetricians and Gynecologists 61st Annual Clinical Meeting in New Orleans May 4-8, 2013. The ClearVue 650 is a lightweight and cost-effective system that spans a range of applications from obstetrics and gynecology to cardiology, abdominal, vascular, breast, musculoskeletal, urology and general imaging.
UltraSPECT has announced its distribution agreement with PharmaLogic for the sale of UltraSPECT’s cardiac and bone imaging applications. The agreement will enable PharmaLogic to provide its hospitals and imaging centers with access to UltraSPECT’s Xpress line of products for lower radiation dose with no diminished image quality.
Scranton Gillette Communications’ sister publications Imaging Technology News and Diagnostic and Interventional Cardiology were honored with American Society of Healthcare Publication Editors (ASHPE) 2013 awards, in recognition of editorial excellence and achievement in the field of healthcare publishing.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
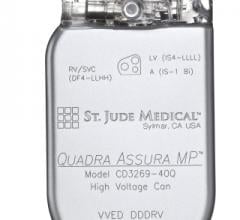
St. Jude Medical Inc., a global medical device company, has announced first enrollment of its MultiPoint Pacing clinical study to build upon its first- to-market Quadripolar Pacing System. Patients will be implanted with the Quadra Assura MP cardiac resynchronization therapy defibrillator (CRT-D) and Quartet lead to assess pacing in multiple locations in the heart.
eCardio Diagnostics announced the launch of eCardio Verite — a patient-friendly, wireless monitor offering seamless transition between mobile cardiac telemetry and cardiac event monitoring when directed by a physician.
Simbionix USA Corporation is proud to announce the release of various training modules for the ANGIO Mentor endovascular simulator.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Zoll Medical said results from the prospective registry and follow-up of Patients Using the Wearable Defibrillator (WEARIT-II) trial will be presented in a late-breaking clinical trial presentation at Heart Rhythm 2013. The session will be presented during the Heart Rhythm Society’s 34th Annual Scientific Sessions, Friday, May 10, 1:30-3 p.m., session number SP22.
InterValve Inc. announced that it has received 510(k) clearance to market the new V8 Aortic Valvuloplasty Balloon Catheter in the United States.
Acutus Medical Inc. (ACM) announced that it has performed its First-In-Man clinical study of its cardiac catheter to provide highest-quality real-time 3-D mapping of the human heart.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
St. Jude Medical Inc. announced CE Mark approval and European launch of its Allure Quadra Cardiac Resynchronization Therapy Pacemaker (CRT-P). This CRT-P system offers more pacing options to deliver higher success rates, as evidenced by robust clinical data. Allure Quadra CRT-P brings the quadripolar lead technology to the pacemaker market. Quadripolar leads allow for increased implant efficiencies, which clinical data indicates can result in fewer surgical revisions. The Allure family of devices also offers heart failure (HF) diagnostics, including CorVue Impedance Monitoring, for improved patient management.
The U.S. Food and Drug Administration approved Kcentra (prothrombin complex concentrate, human) for the urgent reversal of vitamin K antagonist (VKA) anticoagulation in adults with acute major bleeding.
Royal has completed patient enrollment in the CHILL-MI clinical study designed to further evaluate the safety and effectiveness of the company’s InnerCool RTx endovascular cooling system in patients with ST-elevation myocardial infarction (STEMI).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Cook Medical has initiated a global voluntary recall of its Zilver PTX Drug Eluting Peripheral Stent based on its investigation into a small number of complaints that the delivery system of the device had separated at the tip of the inner catheter. Cook received 13 complaints of delivery system tip separation with an occurrence rate of 0.043 percent. Two adverse events, including one death, occurred in cases where a tip separation was reported.
The first patient in the United States has been implanted with the Boston Scientific Corporation ImageReady MR Conditional pacing system in the SAMURAI clinical trial. The study is designed to confirm the safety and effectiveness of the system in the magnetic resonance imaging (MRI) environment. While MRI images can help clinicians make informed decisions about treatment and care, most pacemakers are not compatible with MRI technology, and therefore patients may not have access to the sophisticated scanning capabilities of the diagnostic system.
Agfa HealthCare announced that Hunt Regional Healthcare, Greenville, Texas, has upgraded its Agfa HealthCare picture archive and communication system (PACS) to Impax 6.5 and installed Agfa HealthCare's Cardiology PACS and Xero Viewer to deliver a consolidated view and centralized management of patient, image and information data. With the addition of Agfa HealthCare's Xero Viewer, physicians are able to access the nearly 100,000 imaging exams and reports done each year, on virtually any Hunt Regional facility device. Because the information is web-based, physicians can access the results in real time.


 May 08, 2013
May 08, 2013