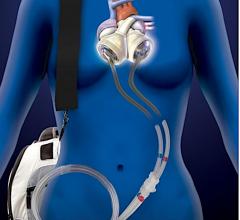
April 23, 2020 – LivaNova PLC said the U.S. Food and Drug Administration (FDA) is now permitting the company to expand ...
Cardiovascular Surgery
This channel includes news and new technology innovations for cardiac surgery. This includes coronary artery bypass grafts (CABG) and surgical valve replacements.

April 7, 2020 — The U.S. Food and Drug Administration (FDA) has issued guidance to provide a policy to help expand the ...
March 30, 2020 — Patients undergoing coronary bypass graft (CAGB) surgery lived longer and had better outcomes when ...
March 30, 2020 — Patients in the United Kingdom (U.K.) who underwent transcatheter aortic valve replacement (TAVR) did ...
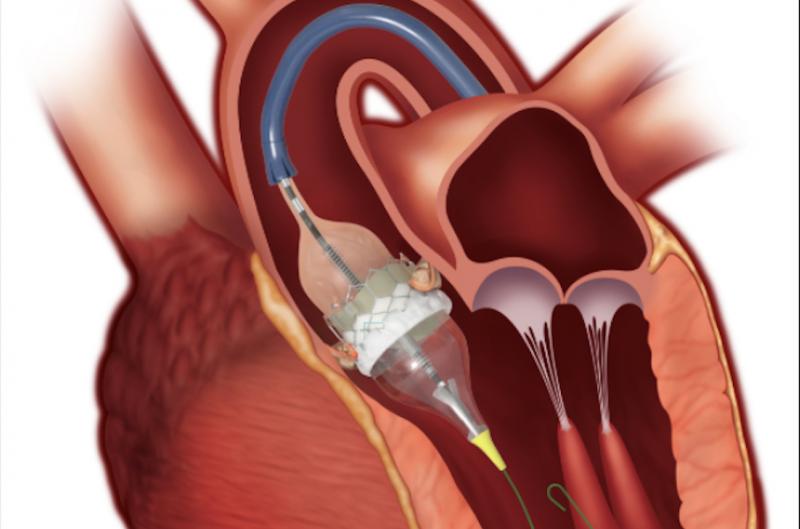
March 30, 2020 — Low-risk patients undergoing transcatheter aortic valve repair (TAVR) at two years did just as well ...
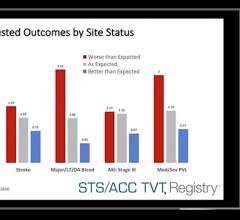
March 29, 2020 — Worse than expected outcomes were found for survival and quality of life among patients undergoing ...

March 29, 2020 — Patients with bicuspid, or two-leaflet, aortic valves who undergo transcatheter aortic valve ...
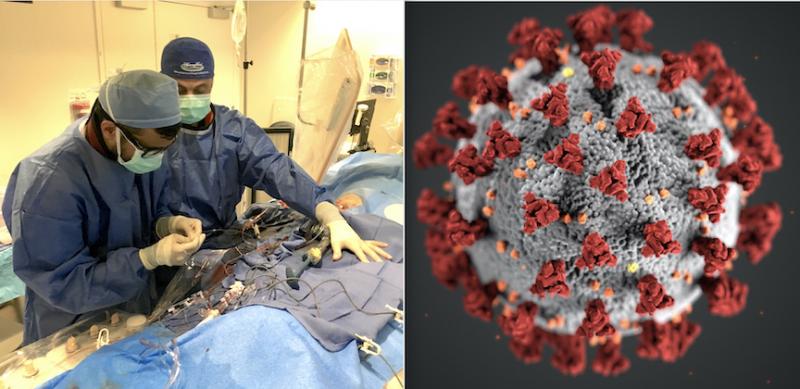
March 20, 2020 — The Centers for Medicare and Medicaid Services (CMS) announced March 18, 2020, that all elective ...
An interview with Ehtisham Mahmud, M.D., FSCAI, chief, Division of Cardiovascular Medicine, executive director of ...
March 10, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the SynCardia Systems 50cc temporary Total ...
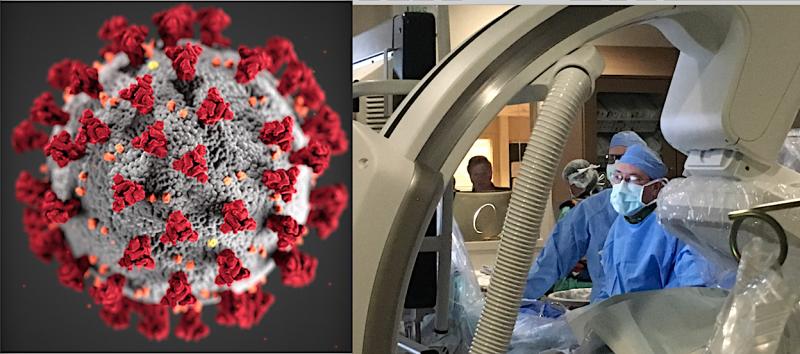
March 9, 2020 — The American College of Cardiology (ACC) is offering updates on the clinical impact of novel coronavirus ...
March 5, 2020 — Abbott recently received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA ...
February 27, 2020 — The U.S. Food and Drug Administration (FDA) sent a safety communication this week to healthcare to ...

In August 2019, the U.S. Food and Drug Administration (FDA) expanded the indication for the use of transcatheter aortic ...
February 12, 2020 — The U.S. Food and Drug Administration (FDA) has approved Carmat's investigational device exemption ...


 April 23, 2020
April 23, 2020