June 5, 2014 — The president of the American College of Cardiology (ACC), Patrick O’Gara, M.D., recently explored what ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

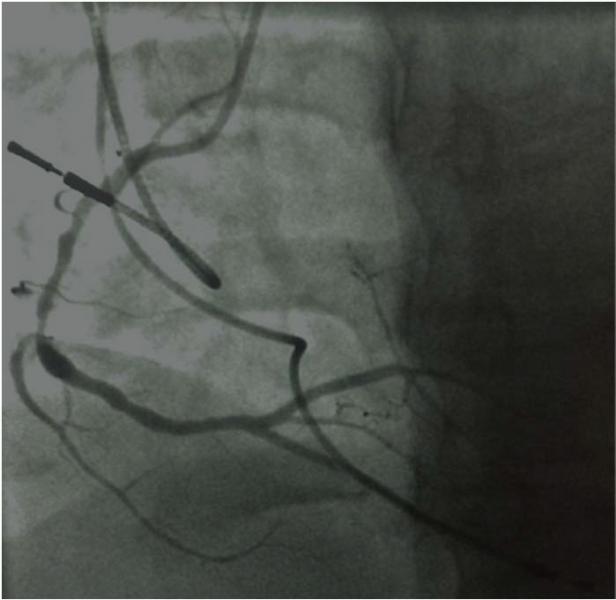
June 5, 2014 — New measures of the severity of coronary artery blockages do not provide enough accuracy to guide ...
June 5, 2014 — Cook Medical is engaged in two clinical studies that will provide additional data on the safety and ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
June 4, 2014 — The Accreditation for Cardiovascular Excellence (ACE) Board of Directors has approved a new set of ...
June 4, 2014 — Biotronik has expanded its line of reinforced introducer sheaths. Following up on the success of the 4 ...
June 3, 2014 — Medtronic Inc. announced CE mark and launch of the NC Euphora noncompliant balloon dilatation catheter ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
June 3, 2014 — Strokes kill nearly 130,000 Americans every year, according to the Centers for Disease Control and ...
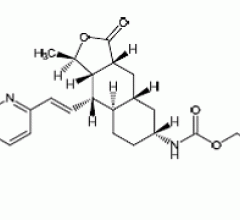
June 3, 2014 — The U.S. Food and Drug Administration (FDA) announced it approved Zontivity (vorapaxar) tablets to reduce ...
June 2, 2014 — As more endovascular medical programs adapt simulation as an essential part of their training curriculum ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
June 2, 2014 — June St. Jude Medical Inc. announced preliminary results from the EnligHTN III clinical study, which ...
May 30, 2014 — The U.S. Food and Drug Administration (FDA) granted market clearance to the CardioMEMS Heart Failure ...

During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
May 27, 2014 — Ekos Corp. announced the U.S. Food and Drug Administration (FDA) has cleared the EkoSonic endovascular ...
May 27, 2014 — Terumo BCT has entered into a business relationship with Kaneka to gain market authorization in the ...
May 23, 2014 — Stentys presented final results from the APPOSITION IV study of its new self-apposing sirolimus-eluting ...

 June 05, 2014
June 05, 2014