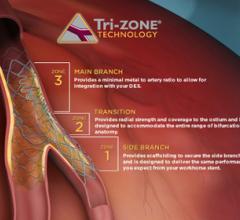
August 21, 2014 — Efforts over the past decade to improve the quality of care for cardiovascular disease patients and ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

August 21, 2014 — Corindus Vascular Robotics announced the results of a retrospective study comparing use of radiation ...
August 21, 2014 — Cardiva Medical Inc. said it closed the first two tranches of a $16.5 million Series 3 private equity ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
August 20, 2014 — AstraZeneca received confirmation from the United States Department of Justice that it is closing its ...
August 20, 2014 — Boston Scientific Corp. and Asahi Intecc have formalized plans to develop a new, differentiated fracti ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
August 19, 2014 — Bluegrass Vascular Technologies closed $4.5 million in Series A financing, that will allow the company ...
August 19, 2014 — At the Transcatheter Cardiovascular Therapeutics (TCT) 2014 meeting Sept. 13-17, Toshiba will display ...
August 18, 2014 — Polidocanol injectable foam (Varithena), the only U.S. Food and Drug Administration (FDA)-approved ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
August 15, 2014 — OrbusNeich announced first patient enrollment in the Randomized Evaluation of short-term Dual anti ...
August 12, 2014 — Teleflex Inc.’s subsidiary Hotspur Technologies Inc. received U.S. Food and Drug Administration (FDA) ...
August 8, 2014 — Biotronik announced the completion of patient enrollment in the superficial femoral artery (SFA) arm of ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
August 7, 2014 — Transluminal Technologies LLC, a Syracuse, NY-based medical device company, received CE mark approval ...
August 7, 2014 — Tryton Medical Inc. announced that the first patient has been enrolled in the EXTENDED Access Registry ...

August 6, 2014 — Acist Medical Systems announced it entered into a strategic agreement with Medtronic to co-promote the ...

 August 21, 2014
August 21, 2014