Nov. 11, 2025 -— Integra LifeSciences Holdings Corp. has announced the FDA 510(k) clearance for use of its CUSA Clarity ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
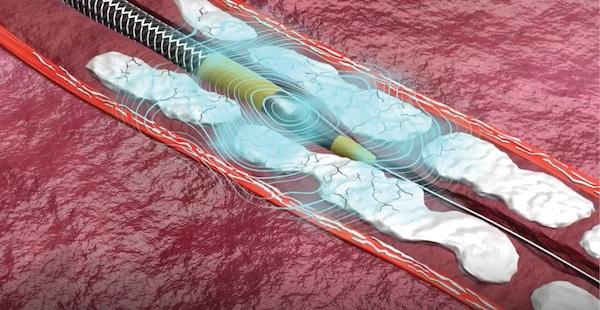
Since receiving FDA approval in 2016, intravascular lithotripsy (IVL) systems have grown in popularity among ...
Nov. 11, 2025 — FastWave Medical has successfully completed enrollment in its 30-patient coronary feasibility study and ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Nov. 10, 2025 — Sentante, a med-tech robotics company, has successfully demonstrated a first-of-a-kind remote stroke ...
Nov. 4, 2025 — Amplitude Vascular Systems (AVS), a medical device company focused on treating calcified arterial disease ...
Nov. 3, 2025 — Penumbra, Inc. has announced additional results of the STORM-PE randomized controlled trial (RCT), which ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Nov. 4, 2025 – Johnson & Johnson MedTech has announced the one-year results in patients treated with its Shockwave ...
Oct. 28, 2025 — Results from the first-of-its-kind randomized PROCTOR trial found that a strategy of saphenous vein ...
Oct. 30, 2025 — Recor Medical and its parent company, Otsuka Medical Devices presented the results from two clinical ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
Oct. 22, 2025 -- Medical robotics innovator Noah Medical presented two new data sets highlighting the clinical and ...
Oct. 29, 2025 — FastWave Medical presented new first-in-human (FIH) and pre-clinical data for its Sola coronary laser ...
Oct. 27, 2025 — At the annual Transcatheter Cardiovascular Therapeutics (TCT 2025) meeting in San Francisco, Royal ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Oct. 27, 2025 — Results from the PREVUE-VALVE study suggest that there are currently at least 4.7 million people aged 65 ...
Oct. 28, 2025 — People who underwent a minimally invasive procedure to have their heart’s aortic valve replaced had ...
Oct., 2025 — Elucid, an AI medical technology company focused on providing physicians with a more precise view of ...


 November 18, 2025
November 18, 2025