July 25, 2025 — Data in recent staffing surveys from the American Society of Radiologic Technologists show that vacancy ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
July 1, 2025 — Stereotaxis, a provider of surgical robotics for minimally invasive endovascular intervention, has ...
July 9, 2025 — InspireMD, Inc., developer of the CGuard Prime carotid stent system for the prevention of stroke ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
July 7, 2025 — Medtronic announced that it has treated the first patient in its SPYRAL GEMINI Pilot program (including ...
June 25, 2025 — Royal Philips and Methodist Hospitals recently announced the healthcare provider’s strategic investment ...
June 16, 2025 – Penumbra, Inc. recently announced the completion of enrollment in the STORM-PE clinical trial. This ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
June 11, 2025 — MorningStar Laboratories, LLC, a developer of precision diagnostic tests that address unmet clinical ...
June 12, 2025 — Viz.ai recently announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for ...
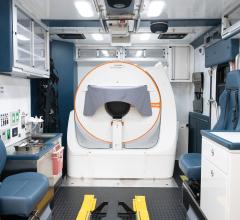
May 28, 2025 — Siemens Healthineers has introduced the first mobile stroke unit (MSU) featuring the Somatom On.site head ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 27, 2025 — Abbott has announced the U.S. Food and Drug Administration (FDA) has approved the company's Tendyne ...
May 27, 2025 — CeleCor Therapeutics has completed its multinational Phase 3 clinical trial of DisaggproT (zalunfiban) ...
May 22, 2025 — Auxira Health has officially launched — the company provides a new approach to clinical support purpose ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
May 21, 2025 — Corify Care recently announced it has entered into a know-how agreement with Mayo Clinic. Corify Care is ...
May 21, 2025 – Johnson & Johnson MedTech has announced the U.S. launch of the Soundstar Crystal Ultrasound Catheter for ...
May 20, 2025 — Shockwave Medical, Inc., part of Johnson & Johnson MedTechhas announced the 30-day primary endpoint ...


 July 25, 2025
July 25, 2025