Oct. 28, 2025 — People who underwent a minimally invasive procedure to have their heart’s aortic valve replaced had ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
Oct., 2025 — Elucid, an AI medical technology company focused on providing physicians with a more precise view of ...
Oct. 25, 2025 — Medtronic plc has announced the launch of the Stedi Extra Support guidewire, designed to enhance ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Oct. 27, 2025 — Elixir Medical, a developer of technologies to treat cardiovascular disease, has announced new clinical ...
Oct. 23, 2025— Emboline, Inc., a provider of full-body embolic protection devices for transcatheter procedures, thas ...
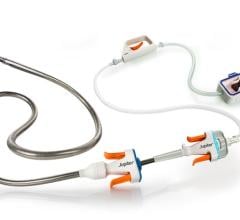
Oct. 26, 2025 – Jupiter Endovascular, Inc., a medical technology company developing a new class of endovascular ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Oct. 27, 2025 – Penumbra, Inc. has announced the results of the STORM-PE randomized controlled trial (RCT), which found ...
Oct. 21, 2025 — SpectraWAVE, Inc., a medical imaging company focused on improving the treatment and outcomes for ...
Oct. 22, 2025 — Heartflow, Inc. has introduced Heartflow PCI Navigator, the newest addition to the Heartflow One ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
Oct. 22, 2025 — Qure has announced its latest (510) K clearance from the US Food and Drug Administration (FDA).
The ...
Oct. 22, 2025 — Nyra Medical, a leading innovator in structural heart therapies, has announced the initiation of its ...
Oct. 15, 2025 — Stereotaxis recently announced it has obtained CE Mark in Europe and submitted a 510(k) application to ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Oct. 13, 2025 — Medtronic plc recently announced it has received U.S. Food and Drug Administration (FDA) labeling ...
Oct. 7, 2025 — Cagent Vascular has announced the commercial launch of the Serranator PTA Serration Balloon Catheter in 7 ...
Oct. 7, 2025 — Medtronic has announced the full distribution of the Neuroguard IEP System (Neuroguard) after a ...


 October 29, 2025
October 29, 2025