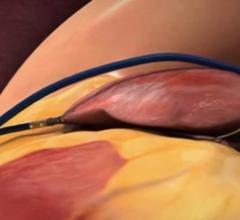
May 15, 2021 - Transcatheter left atrial appendage occlusion (LAAO) with a Boston Scientific Watchman device was ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

May 15, 2021 — Patients with an elevated risk of stroke due to heart rhythm problems, or atrial fibrillation (AFib) ...
May 15, 2021 — The ADAPTABLE trial found no significant differences in cardiovascular events or major bleeding in ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
May 15, 2021 — The anticoagulant apixaban (Eliquis) was not superior to standard of care following transcatheter aortic ...

There is a trend in interventional cardiology that is now being called “renalism,” where patients with poor renal ...

The mitral valve anatomy is extremely complex, which has caused many challenges for transcatheter mitral valve ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Tom Jones, M.D., director, cardiac catheterization laboratories, Seattle Children’s Hospital, and principle investigator ...
May 12, 2021 — Abbott recently announced its new interventional imaging platform powered by Ultreon 1.0 Software, has ...
May 12, 2021 — Preliminary results of a clinical trial, presented today at the AATS 101st Annual Meeting, showed that a ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
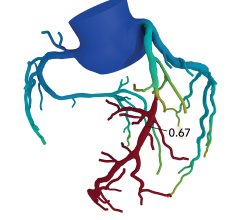
May 12, 2021 — HeartFlow, Inc., a leader in revolutionizing precision heartcare, today announced that the National ...

Heart failure remains an unheralded global pandemic, costing society billions of dollars each year.[1,2] Projections ...
Philippe Géneréux, M.D., director of the structural heart program at Atlantic Health System’s Morristown Medical Center ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Arnold Seto, M.D., MPA, FSCAI, chief of cardiology, Long Beach Veterans Affairs Medical Center and director ...
Payam Dehghani, M.D., FRCPC, FACC, FSCAI, co-director of Prairie Vascular Research and associate professor at the ...
April 29, 2021 — Here is the list of late-breaking study presentations and links to articles about each of them from the ...

 May 16, 2021
May 16, 2021