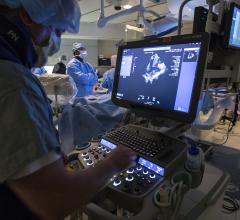
August 25, 2020 – Atlantic Health System’s Morristown Medical Center has opened one of the region’s first radial lounges ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

The cardiology program at Tufts Medical Center in Boston is internationally recognized for being on the forefront of ...
August 21, 2020 — According to a report published by Research Dive, the rising number of patients suffering from atrial ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
August 18, 2020 — Philips Healthcare introduced its new OmniWire, the world’s first solid core fractional flow reserve ...
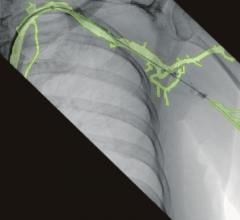
August 18, 2020 — Ziehm Imaging announces the acquisition of Therenva, a French-based developer of planning and imaging ...
August 17, 2020 — Veryan Medical announced it will support Vasorum in the commercialization of the Celt atrial closure ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Advanced visualization company Medis recently purchased Advanced Medical Imaging Development S.r.l. (AMID), which ...
August 10, 2020 - The Guerin Family Congenital Heart Program in the Smidt Heart Institute at Cedars-Sinai is expanding ...

Drug-coated balloons (DCB), also referred to as drug-eluting balloons (DEB), were created as a way to reduce very high ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
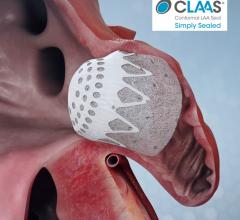
August 6, 2020 – Conformal Medical Inc. announced it secured $85 million in Series C financing to support the company’s ...
August 5, 2020 — The U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) this week for ...
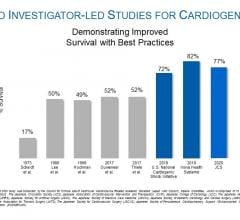
August 3, 2020 — A three-year, investigator-led, prospective study of Japanese patients who received an Impella heart ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

August 3, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) mag ...
Devi G. Nair, M.D., FHRS, director of cardiac electrophysiology, St. Bernards Heart and Vascular Center, Jonesboro, Ark ...
July 24, 2020 — CeloNova BioSciences Inc. announced it successfully completed enrollment of the COBRA REDUCE randomized ...


 August 25, 2020
August 25, 2020