July 22, 2015 — Heart failure patients had a significantly lower chance of being readmitted within 30 days of discharge ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
July 22, 2015 Direct Flow Medical Inc. received Investigational Device Exemption (IDE) approval from the U.S. Food and ...

July 22, 2015 — St. Jude Medical and Thoratec announced that the boards of directors of both companies have unanimously ...
July 21, 2015 — The recent U.S. Food and Drug Administration (FDA) approvals of two next-generation transcatheter aortic ...

July 17, 2015 - Hospitals in the Midwest were more likely than others to refer patients for guideline-recommended cardia ...

July 17, 2015 - Building teams that include advanced practice providers can help cardiovascular practices meet the ...
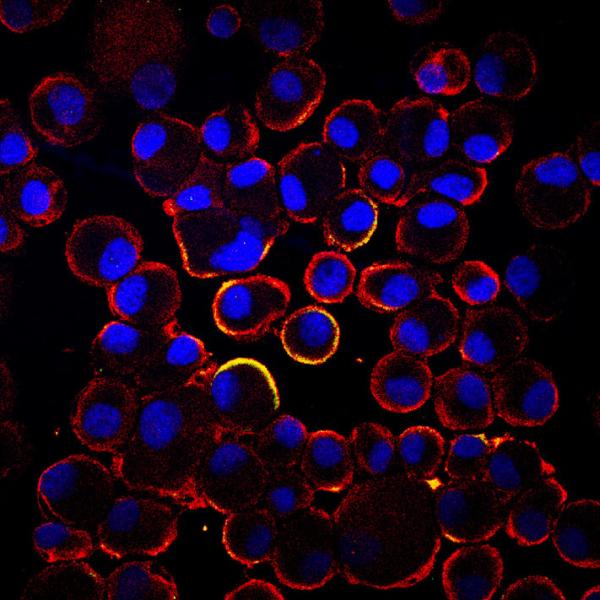
July 17, 2015 - NeoStem Inc. presented updated efficacy and safety results from the one-year follow-up for its Phase 2 ...
July 15, 2015 - Vital Images Inc. will debut its U.S. Food and Drug Administration (FDA) 510(k)-cleared CT Myocardial ...
July 15, 2015 - Konica Minolta Medical Imaging announced new Blue Moon Lifecycle Solutions designed to help customers ...
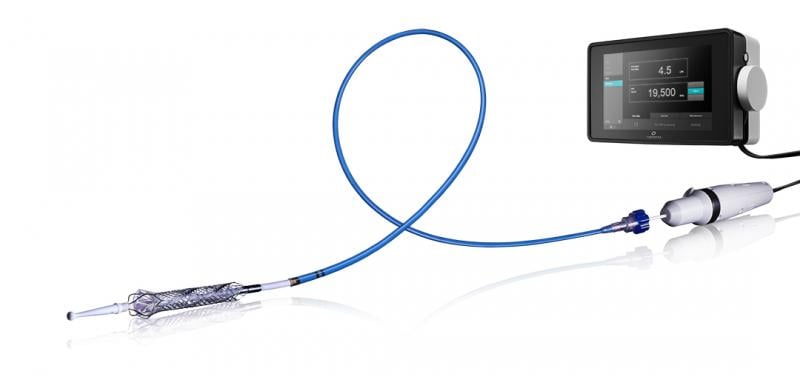
July 15, 2015 - Thoratec announced that the HeartMate PHP (Percutaneous Heart Pump) received CE Mark approval ...
July 14, 2015 - Biotronik announced the completion of the BIOVALVE first-in-human trial for its new transcatheter aortic ...
July 14, 2015 - Mindray announced the release of its TE7 Touch Enabled Ultrasound System, which supports rapid and ...
July 14, 2015 - On July 8, the Centers for Medicare and Medicaid Services (CMS) released the first proposed update to ...
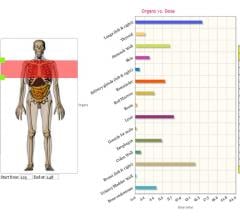
July 13, 2015 - PHS Technologies Group LLC, a division of PACSHealth LLC, announced that it will integrate VirtualDose ...

July 13, 2015 - Edwards Lifesciences Corp. announced that it has agreed to acquire CardiAQ Valve Technologies Inc., a ...


 July 22, 2015
July 22, 2015