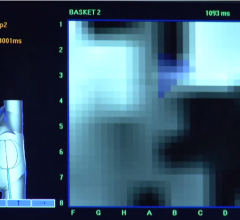
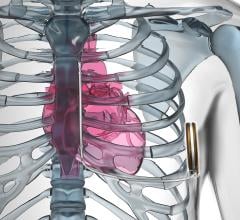
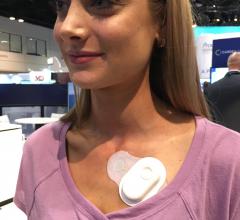
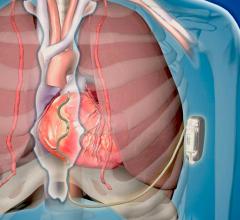
May 15, 2019 — A pilot trial has shown His pacing in cardiac resynchronization therapy (CRT) has been shown to ...
HRS
This channel contains news about the Heart Rhythm Society (HRS), including coverage of its annual meeting and links to recently released practice guidelines. The HRS is a leading resource on cardiac pacing and electrophysiology.
May 14, 2019 – The first prospective, multicenter, randomized, controlled trial to compare conventional pulmonary vein ...
May 14, 2019 — The Heart Rhythm Society (HRS) in partnership with three other professional societies issued an ...
New patient monitoring technologies with wireless connectivity have enabled a revolution in cardiac event and Holter ...
May 14, 2019 — The Heart Rhythm Society (HRS) released a first-of-its-kind consensus statement with guidance on the ...
May 14, 2019 – Results from new research show that passengers with cardiac implantable electronic devices (CIEDs), such ...
May 13, 2019 — Boston Scientific announced acute results from the UNTOUCHED study evaluating safety and efficacy of the ...
The show floors at the American College of Cardiology (ACC) 2018 meeting in March and at the Heart Rhythm Society (HRS) ...
May 13, 2019 – Results from a new survey are the first to report a large discrepancy in patient’s knowledge of their ...
May 13, 2019 – A first-in-human pilot study of Medtronic's investigational Extravascular Implantable Cardioverter ...
May 13, 2019 — A randomized clinical trial effectively used nerve stimulation through an ear clip to reduce atrial ...
May 13, 2019 — A digital screening for atrial fibrillation (AFib or AF) successfully monitored more than 60,000 ...
May 13, 2019 — BioTrace Medical Inc. announced the company’s Tempo Lead has obtained CE Mark certification in Europe for ...
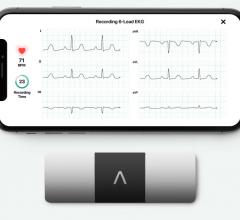
May 13, 2019 — AliveCor announced its third U.S. Food and Drug Administration (FDA) clearance in three months, making ...
May 13, 2019 — Imricor announced the signing of a commercial agreement with the Haga Hospital in The Hague, Netherlands ...
May 10, 2019 — Spacelabs Healthcare debuted Sentinel 11 at the 40th Annual Heart Rhythm Society (HRS) Scientific ...
May 9, 2019 – Stereotaxis introduced Stereotaxis Genesis RMN, its next-generation robotic platform that it claims is a ...


 May 15, 2019
May 15, 2019