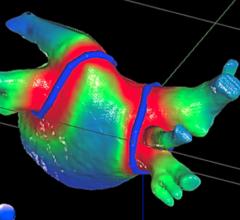
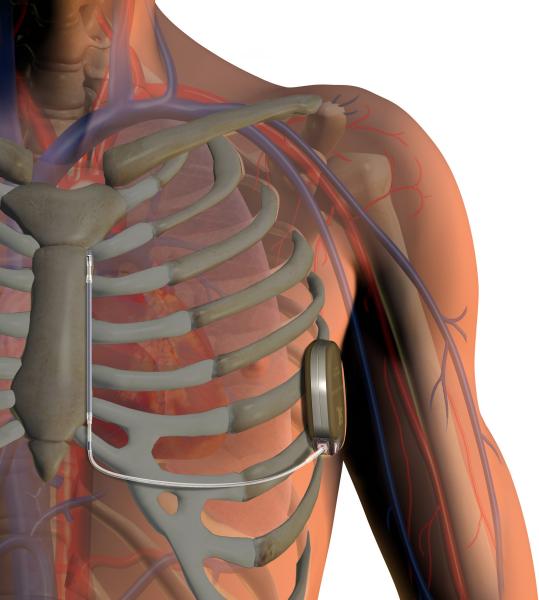
May 13, 2020 — Cardiac resynchronization therapy (CRT) using biventricular pacing (BVP) or His bundle pacing (HBP) is ...
HRS
This channel contains news about the Heart Rhythm Society (HRS), including coverage of its annual meeting and links to recently released practice guidelines. The HRS is a leading resource on cardiac pacing and electrophysiology.
May 13, 2020 — The PRECEPT study testing the use of the Biosense Webster Thermocool Smarttouch SF Catheter for the ...
May 13, 2020 — The patient-administered nasal spray drug etripamil did not meet its primary endpoint in treating ...
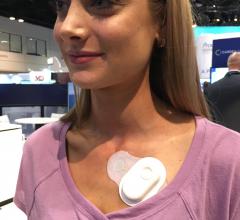
New patient monitoring technologies with wireless connectivity have enabled a revolution in cardiac event and Holter ...
May 8, 2020 — Final results from the UNTOUCHED study of the Emblem Subcutaneous Implantable Defibrillator (S-ICD) System ...
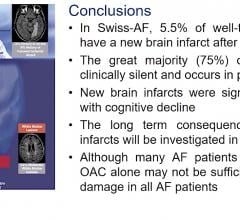
May 8, 2020 – A new clinical study found that patients with atrial fibrillation (AF) experienced a high incidence of ...
May 8, 2020 — Positive 12-month results were announced today from the PINNACLE FLX clinical trial assessing the safety ...
The show floors at the American College of Cardiology (ACC) 2018 meeting in March and at the Heart Rhythm Society (HRS) ...
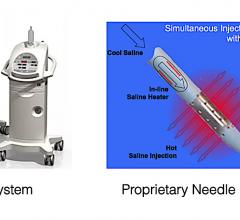
May 8, 2020 – Results from a first-in-human early feasibility study (EFS) using a saline enhanced radiofrequency (SERF) ...
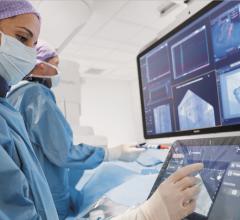
May 8, 2020 — New clinical trial reveals the first-in-human results for paroxysmal or persistent atrial fibrillation (AF ...
May 8, 2020 — A new clinical trial is the first to compare the safety and efficacy of subcutaneous implantable ...
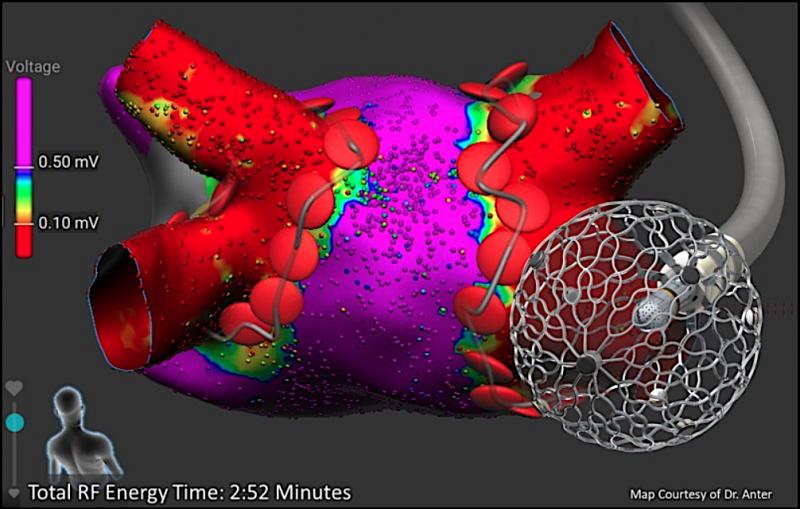
May 8, 2020 — A new clinical trial effectively uses pulsed field (PF) energy to treat patients with persistent or ...
May 1, 2020 — The Heart Rhythm Society (HRS) has created a COVID-19 Task Force to make recommendations related to ...

April 8, 2020 – The scientific community is learning more about the impact and interaction of cardiovascular diseases ...

April 1, 2020 — Due to the continued global escalation of the novel coronavirus (COVID-19, SARS-CoV-2), the Heart Rhythm ...
September 17, 2019 — Treating high-risk heart patients with a single, high dose of radiation therapy can dramatically ...
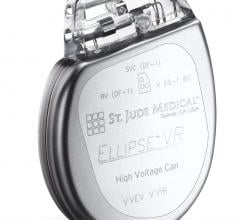
August 5, 2019 — Abbott is recalling the Ellipse Implantable Cardioverter Defibrillators (ICDs) because electrical ...


 May 13, 2020
May 13, 2020