Pierre Qian, MBBS, cardiac electrophysiologist fellow, Brigham and Women's Hospital, explains how his facility is ...
HRS
This channel contains news about the Heart Rhythm Society (HRS), including coverage of its annual meeting and links to recently released practice guidelines. The HRS is a leading resource on cardiac pacing and electrophysiology.

This is a brief overview of updates on implantable cardioverter defibrillators (ICD), including new technology ...
May 17, 2019 — Digital healthcare company Murj announced the availability of the Murj Analytics software-as-a-service ...
New patient monitoring technologies with wireless connectivity have enabled a revolution in cardiac event and Holter ...
May 17, 2019 — At the 40th annual Heart Rhythm Scientific Sessions, May 8-11 in San Francisco, Acutus Medical announced ...
May 16, 2019 — Preventice Solutions presented clinical data validating its BodyGuardian Remote Monitoring System with ...
May 16, 2019 — Johnson & Johnson Medical Devices Companies announced the launch of Biosense Webster Inc.’s Cartonet to ...
The show floors at the American College of Cardiology (ACC) 2018 meeting in March and at the Heart Rhythm Society (HRS) ...
The Heart Rhythm Society (HRS) 2019 Annual Scientific Sessions represent an important annual event for electrophysi ...
May 16, 2019 — Johnson & Johnson Medical Devices Companies announced that Biosense Webster, Inc.’s QDot Micro catheter ...
May 16, 2019 — CardioFocus Inc. announced the presentation of results from its pivotal confirmatory study evaluating the ...
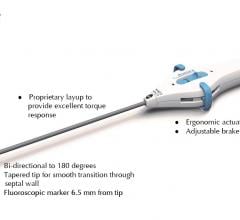
May 16, 2019 — BioCardia Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the Avance steerable ...

May 15, 2019 — The Heart Rhythm Society (HRS) had 21 late-breaking study presentations at the 2019 Heart Rhythm ...

May 15, 2019 — A new study shows electromechanical wave imaging is capable of localizing arrhythmias including atrial ...
May 15, 2019 — A new infection risk scoring system has been developed based on data from the large PADIT Trial.[1] The ...
May 15, 2019 — Renal artery denervation (RDN) was found to be safe when employed with pulmonary vein isolation (PVI) to ...
May 15, 2019 - Three new studies show that patients who are medically indicated for implantable heart devices, including ...

 July 25, 2019
July 25, 2019




![A new infection risk scoring system has been developed based on data from the large PADIT Trial.[1] The new scoring system was presented as a follow up to that study during a late-breaking session at Heart Rhythm 2019, the Heart Rhythm Society's 40th Annual Scientific Sessions.](/sites/default/files/styles/content_feed_medium/public/PADIT_Infection_Risk_score.jpg?itok=O1-YAcMm)