March 28, 2023 — The Society for Cardiovascular Angiography and Interventions (SCAI) and the Heart Rhythm Society (HRS) ...
HRS
This channel contains news about the Heart Rhythm Society (HRS), including coverage of its annual meeting and links to recently released practice guidelines. The HRS is a leading resource on cardiac pacing and electrophysiology.

Hospitals in the U.S. are suffering under a debilitating post-pandemic financial and operational trauma. Ascension poste ...
August 11, 2022 — The Heart Rhythm Society (HRS) announced the full lineup of speakers and sessions for its first annual ...
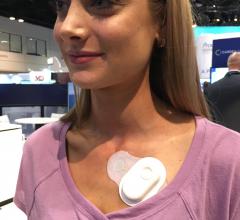
New patient monitoring technologies with wireless connectivity have enabled a revolution in cardiac event and Holter ...
August 4, 2022 — The Heart Rhythm Society (HRS) is a professional society representing a diverse population of ...
May 18, 2022 — A new app developed by the Mayo Clinic transmits Apple Watch electrocardiograms (ECG) signals recorded in ...
May 18, 2022 — Continuous monitoring with an insertable cardiac monitor (ICM) is shown to be more successful in ...
The show floors at the American College of Cardiology (ACC) 2018 meeting in March and at the Heart Rhythm Society (HRS) ...
May 17, 2022 — Results of a new study reveal Black patients hospitalized with atrial fibrillation (AF) are under ...
May 17, 2022 — Results from a new study show success of intended same day discharge (SDD) improves over time for ...
May 17, 2022 — Heart Rhythm 2022 has come to a close, and the Heart Rhythm Society has released some stats regarding ...
May 4, 2022 – Single-lead ECG tracings from an Apple Watch interpreted by an artificial intelligence (AI) algorithm ...
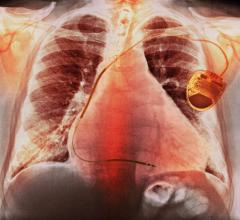
May 2, 2022 – High-risk patients who need defibrillators to prevent cardiac arrest can experience fewer complications ...
April 29, 2022 – Medtronic has announced new data from the STROKE AF clinical trial, which showed that large and small ...
April 29, 2022 – Vektor Medical has announced positive results from its “Vektor vMap Clinical Validation Study” ...
Khaldoun Tarakji, M.D., MPH, associate section head, cardiac electrophysiology, Heart and Vascular Institute at ...
August 9, 2021 - In association with Heart Rhythm 2021, Biotronik today announced that the latest implantable cardiac ...

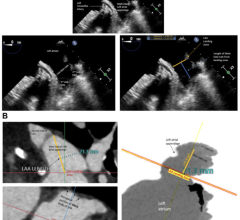
 March 28, 2023
March 28, 2023