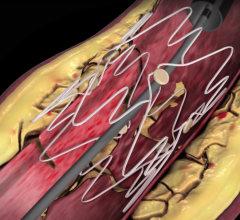
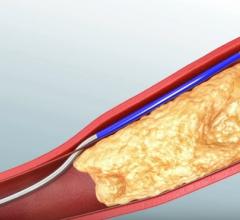
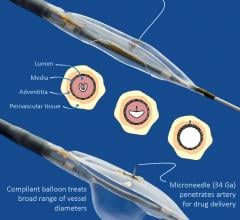
Aug. 1, 2017 — LimFlow SA announced publication of positive results from the pilot study of the LimFlow Percutaneous ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
July 26, 2017 — The Spectranetics Corp. announced receipt of U.S. Food and Drug Administration (FDA) pre-market approval ...
July 26, 2017 — Intact Vascular Inc. recently announced that its Tack Optimized Balloon Angioplasty II Below the Knee ...
This video case study, provided by Gore Medical, is titled "Tackling Complex Cases in Dialysis Access," by John Ross, M ...
July 17, 2017 — LimFlow SA announced enrollment of the first patient in the U.S. feasibility study of the LimFlow ...
July 14, 2017 — Intact Vascular Inc. announced the U.S. Food and Drug Administration (FDA) approved an Investigational ...
June 28, 2017 — Royal Philips recently announced the relaunch of its Pioneer Plus catheter, the first and only re-entry ...
June 26, 2017 — Shockwave Medical recently announced two milestones for its Lithoplasty System for the treatment of ...
June 12, 2017 – Med Alliance announced completion on schedule of patient enrollment in the first-in-man (FIM) study of ...
May 30, 2017 — The U.S. Food and Drug Administration (FDA) announced that it has granted market clearance to Ra Medical ...
May 17, 2017 — Contego Medical LLC announced recently that it has received CE Marking of its Vanguard IEP Peripheral ...
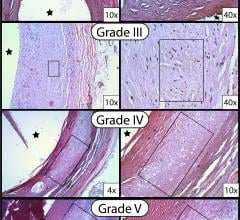
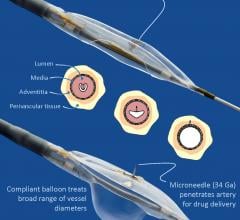
May 12, 2017 — Peripheral artery disease (PAD) patients who were treated with an anti-inflammatory steroid injected ...
May 3, 2017 — A new study published in The Anatomical Record finds that peripheral arteries, easily accessible by ...
April 28, 2017 — Biotronik’s Pulsar-18 bare metal stent (BMS) has yielded high primary patency in a real-world setting ...
April 27, 2017 — Mercator MedSystems Inc. announced the first patient enrollment into the TANGO (Temsirolimus ...


 August 02, 2017
August 02, 2017