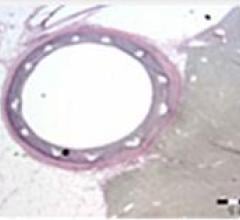
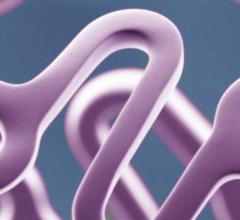
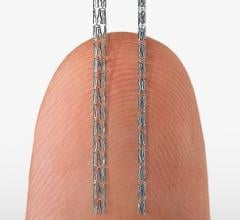
March 16, 2015 — Medtronic plc unveiled the preclinical outcomes of its novel Drug-Filled Stent (DFS) at the 64th Annual ...
Stents Drug Eluting
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
March 6, 2015 — A three-year review of data on the Combo Dual Therapy Stent was presented at the Joint Interventional ...
February 17, 2015 — Micell Technologies, Inc. announced the commercial availability of the MiStent sirolimus-eluting abs ...
The America College of Cardiology has released its list of key late-breaking clinical trials at the ACC 2015 meeting in ...
January 5, 2015 — InspireMD, Inc. announced results from two important clinical trials. Thirty-day results from the ...

December 10, 2014 — In the first successful United States trial of a bioabsorbable polymer stent, the Boston Scientific ...

Nov. 13, 2014 — Optimizing the treatment of coronary artery disease with a new foundation for future stent innovations ...
November 4, 2014 — The U.S. Food and Drug Administration (FDA) has cleared the Abbott family of Xience everolimus ...
October 20, 2014 — Boston Scientific Corp. has initiated the PLATINUM Diversity trial to evaluate the clinical ...
September 25, 2014 — Svelte Medical Systems reported that its drug-eluting coronary stent Integrated Delivery System ...


 March 16, 2015
March 16, 2015