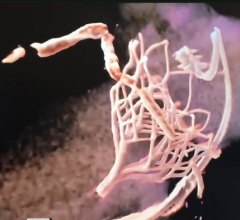
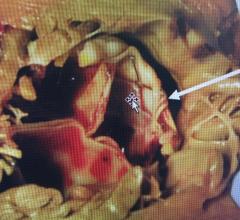
A discussion with William O'Neill, M.D., director of the structural heart program, Henry Ford Hospital, and Michele ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
September 5, 2019 — Abbott announced the launch of the company's TRILUMINATE Pivotal trial evaluating the safety and ...
September 3, 2019 — Shockwave Medical Inc. has received Breakthrough Device Designation from the U.S. Food and Drug ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...
September 3, 2019 — Corindus Vascular Robotics Inc. announced that EClinicalMedicine, a clinical journal published by Th ...
August 29, 2019 – Merit Medical Systems Inc. announced the U.S. commercial launch of the PreludeSync Evo radial ...

August 28, 2019 — The U.S. Food and Drug Administration (FDA) released an updated MedWatch Alert this month on the ...
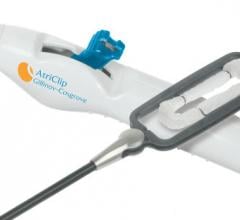
August 28, 2019 — AtriCure Inc. announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance of ...
August 27, 2019 — Concept Medical was recently granted Breakthrough Therapy designation by the U.S. Food and Drug ...
August 21, 2019 — Increasing incidence of cardiovascular diseases worldwide is creating growth opportunities for the ...
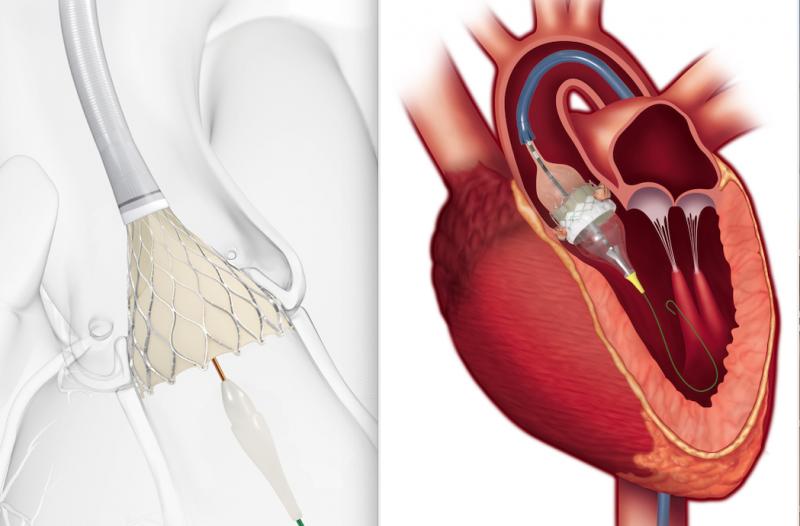
August 16, 2019 — In one coordinated move, the U.S. Food and Drug Administration (FDA) opened use of transcatheter ...
August 8, 2019 — Corindus Vascular Robotics announced it has entered into a definitive merger agreement to be acquired ...
July 17, 2019 — Abbott has received U.S. Food and Drug Administration (FDA) approval for the most advanced MitraClip hea ...
July 9, 2019 — Neovasc Inc. announced that its Tiara transcatheter mitral valve replacement (TMVR) device and its ...
July 3, 2019 — A study of a procedure developed at Henry Ford Health System shows inducing a heart attack in patients ...
June 20, 2019 – VEITHsymposium and the Cardiovascular Research Foundation (CRF) announced an alliance between Transcathe ...


 September 12, 2019
September 12, 2019