June 13, 2019 — Orchestra BioMed Inc. announced it has formed a global strategic partnership with Terumo Corp. for ...
TCT
This channel contains news about the annual Transcatheter Cardiovascular Therapeutics (TCT) conference presented by the Cardiovascular Research Foundation (CRF). It includes coverage from the annual meeting and links CRF news. TCT is the premier conference for the subspecialty of interventional cardiology, including the new subspecialty areas of transcatheter structural heart procedures.
June 10, 2019 – W. L. Gore & Associates (Gore) announced the U.S. Food and Drug Administration’s (FDA’s) premarket ...
May 15, 2019 — Cordis, a Cardinal Health company, recently announced the full U.S. launch of its Radial 360 portfolio ...
The optimal delivery of cardiac care is evolving rapidly. A growing number of patients combined with innovative new ...

April 23, 2019 — The U.S. Food and Drug Administration (FDA) has cleared the Boston Scientific Lotus Edge transcatheter ...
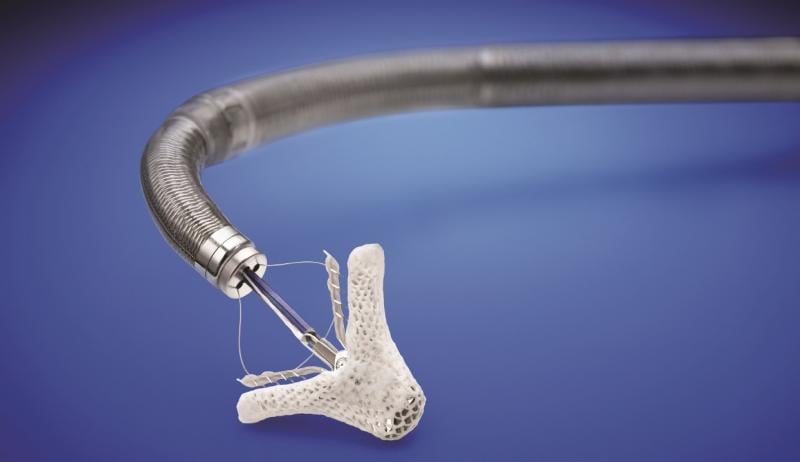
March 14, 2019 — The U.S. Food and Drug Administration (FDA) approved the MitraClip heart valve repair device for ...

February 22, 2019 — The U.S. Food and Drug Administration (FDA) has approved the Biotronik Orsiro drug-eluting stent ...

In recent years, there has been a lot of focus by vendors on developing better stenting technologies to treat peripheral ...
January 2, 2019 — Edwards Lifesciences Corp. announced that the Sapien 3 Ultra system has received U.S. Food and Drug ...
In 2009, the GuideLiner Catheter revolutionized the concept of guide extension, creating new possibilities in ...
October 17, 2018 — Shimadzu Medical Systems USA and Change Healthcare have entered a partnership through which Shimadzu ...
There is a hype cycle surrounding new technologies in all industries, but medicine is unique because of its focus on ...
From a cardiovascular device innovation standpoint, the 2018 Transcatheter Cardiovascular Therapeutics (TCT) conference ...
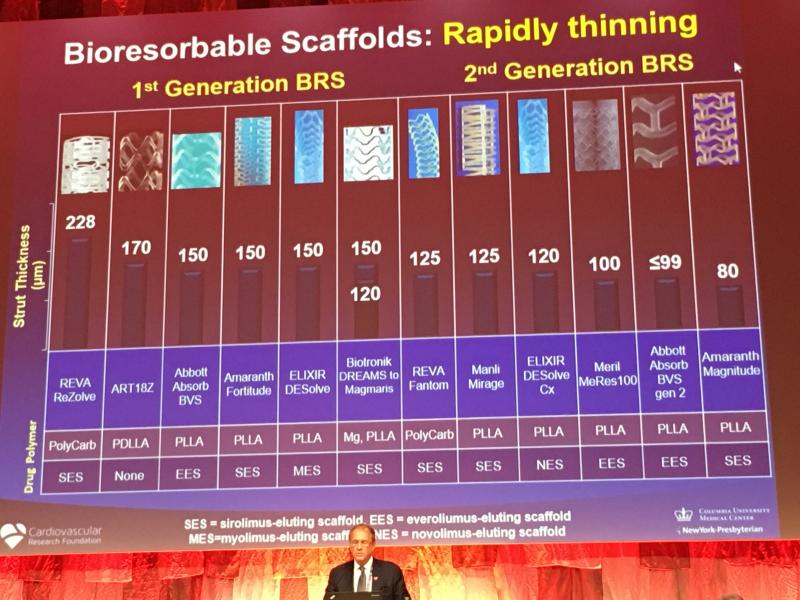
Bioresorbable stent (BRS) technology is not dead, but the unbridled enthusiasm seen two years ago for the technology has ...
October 12, 2018 – Reva Medical presented four key data sets demonstrating the capabilities of the company’s Fantom bior ...
Professor Ian Meredith, MBBS, Ph.D., global chief medical officer and executive vice president, Boston Scientific ...


 June 13, 2019
June 13, 2019