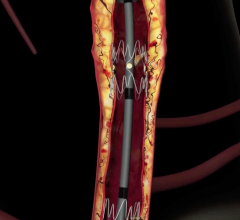
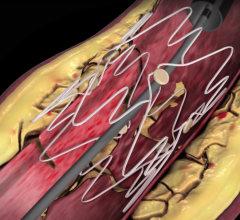
September 28, 2015 — New 12-month clinical trial outcomes assessing the safety and performance of the Boston Scientific Eluvia drug-eluting vascular stent system reflect a primary patency rate of more than 96 percent. These results represent the highest 12-month primary patency reported for an interventional treatment of femoropopliteal artery lesions among comparable trials. The Eluvia Stent System is an advanced treatment option for patients with narrowing or blockages in the superficial femoral artery (SFA) or proximal popliteal artery (PPA), a result of peripheral artery disease (PAD).
Results from the MAJESTIC trial — which were presented at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual meeting in Lisbon, Portugal — also included a low 12-month target lesion revascularization (TLR) rate of 3.8 percent, with no observed stent fractures and no amputations.
"Achieving a 96 percent primary patency rate at one year, with low major adverse events, is exceptional," said Prof. Stefan Muller-Hulsbeck, M.D., PhD, principal investigator and chairman, Vascular Center Diako Flensburg and head of the Department of Diagnostic and Interventional Radiology /Neuroradiology, Academic Hospitals Flensburg, Germany. "In my opinion, the sustained release of paclitaxel enabled by this technology could represent a significant advancement in the treatment of patients with peripheral arterial disease."
The MAJESTIC trial, a prospective, multicenter clinical trial, enrolled 57 patients across Europe, Australia and New Zealand, with an average lesion length of 70.8 mm. The trial included a high percentage of complex lesions, with 46 percent of lesions classified as total occlusions and 65 percent identified as severely calcified.
Data from the MAJESTIC trial are expected to support global regulatory submissions. In addition, Boston Scientific has received an Investigational Device Exemption (IDE) from the U.S. Food and Drug Administration (FDA) to begin a global, pivotal study of the Eluvia Stent System. The study, named the IMPERIAL trial, designed to support future regulatory submissions and patient enrollment, is expected to begin in the coming months.
The Eluvia Stent System is the first stent specifically designed for deployment in the SFA that utilizes the anti-restenotic drug paclitaxel in conjunction with a polymer. This drug and polymer combination is intended to facilitate sustained release of the drug over the period of time when restenosis is most likely to occur, preventing tissue growth that might otherwise block the stented artery. The Eluvia Stent System is built on the Innova Stent System platform, consisting of a self-expanding nitinol stent and an advanced, 6F low-profile triaxial delivery system for added support and placement accuracy.
For more information: www.bostonscientific.com


 November 06, 2019
November 06, 2019