Current ACCF/AHA guidelines recommend fibrinolysis (FL) as the preferred reperfusion strategy for ST-segment elevation myocardial infarction (STEMI) patients with expected delays of > 120 minutes from first medical contact to percutaneous coronary intervention (PCI). The guidelines recommend this be followed by transfer to a PCI center with angiography/PCI within 3-24 hours. However, assessment of reperfusion prior to angiography may not be accurate based on clinical and electrocardiogram (ECG) criteria alone. Recent data suggested increased recurrent ischemic events within the first 24 hours. The aim of this study, which was conducted from 2003-2015, was to assess the safety of very early PCI (< 3 hours) following FL.
The recent, sudden death of a 13-year-old boy resulted in more than 20 relatives being incorrectly diagnosed as having a potentially lethal heart rhythm condition. This erroneous diagnosis occurred as a result of inappropriate use of genetic testing and incorrect interpretation of genetic test results, according to Mayo Clinic research published in Mayo Clinic Proceedings.
November 9, 2016 — Merit Medical Systems Inc. announced that it has received 510(k) clearance for the SwiftNINJA ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
November 9, 2016 — A pioneering way to access the heart for transcatheter valve replacement has a 98 percent success ...
A discussion with Michael Reardon, M.D. about the state of transcatheter aortic valve replacement (TAVR) as of late 2016 ...
November 9, 2016 — A dedicated stent study conducted exclusively in women and minority patients evaluated clinical ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Patients who undergo minimally invasive heart valve replacement, known as transcatheter aortic valve replacement (TAVR), sometimes develop heart rhythm problems that necessitate placement of a permanent pacemaker. However, when a pacemaker is needed soon after TAVR, patients often have worse outcomes than those who did not need a pacemaker, according to a study published recently in JACC: Cardiovascular Interventions. The study shows that the risks are both short- and long-term and include lengthier hospital and intensive care unit stays as well as a greater risk of death.
Juan Granada, M.D., executive director and chief scientific officer of the Cardiovascular Research Foundation's Skirball ...
Brijeshwar Maini, M.D., and Brian Bethea, M.D., from Tenet Florida’s structural heart program, explain the importance of ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
November 9, 2016 – Results from a randomized, multicenter trial failed to show non-inferiority of hybrid, ultra-thin ...
Krishna Rocha-Singh, M.D., Prairie Vascular Institute, Springfield, Ill., explains advancements in device therapy for ...
David Kandzari, M.D., director of interventional cardiology and chief scientific officer, Piedmont Heart Institute ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Studies have shown transcatheter aortic valve replacement (TAVR) has an increased risk of stroke and cerebral damage due ...
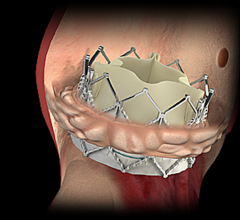
Edwards Lifesciences Corp. recently announced new data demonstrating dramatic and sustained improvements in quality of life for severe aortic stenosis (AS) patients at intermediate surgical risk treated with Edwards transcatheter heart valves. Study results were presented at the 28th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, in Washington, D.C.

November 7, 2016 – Results from the U.S. real-world, post-FDA approval experience of the Watchman device found high ...

 November 10, 2016
November 10, 2016