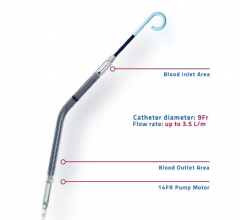
December 19, 2016 — BioTrace Medical Inc. announced the first commercial use of the company’s Tempo Temporary Pacing ...
December 13, 2016 — Coupling data mining of adverse event reports and electronic health records with targeted laboratory ...
Lombard Medical Inc. announced that its Altura endovascular stent graft system was featured in a scientific presentation at the 43rd annual VEITHsymposium, Nov. 15-19 in New York City. The Altura stent graft, which was launched commercially in Europe earlier in 2016, is specifically designed to simplify treatment in patients with normal abdominal aortic aneurysm (AAA) anatomy.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Biotronik announced the presentation of data confirming the efficacy of the Pulsar-18 bare metal self-expanding stent (BMS SE) at VEITHsymposium 2016, Nov. 15-19 in New York. Jos C. van den Berg, University of Bern, Switzerland, presented the encouraging interim results for Pulsar-18 in treatment of superficial femoral artery (SFA) disease during the symposium’s main program on behalf of lead investigator for the BIOFLEX PEACE all-comers trial, Michael Lichtenberg, Vascular Center, Arnsberg, Germany.
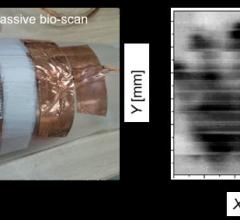
Scientists at Tokyo Institute of Technology have developed a portable and wearable terahertz scanning device for non-invasive inspection of three-dimensional objects. The device is made using arrays of carbon nanotubes and does not require bulky peripheral optical components.
The primary goal of any healthcare provider is to improve the lives of patients through effective treatment. However, because they are also businesses, hospitals have concerns that entail much more than this. To be viable in the long term, hospitals must manage their margins to fund their mission.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
LimFlow SA announced in November that it received the CE Mark for its fully percutaneous LimFlow System designed for venous arterialization of the lower limbs in end-stage patients at risk of limb amputation for critical limb ischemia (CLI).
St. Jude Medical Inc. announced results of the MOMENTUM 3 U.S. IDE Clinical Study during a late-breaking clinical trial session at the American Heart Association (AHA) Scientific Sessions, Nov. 12-16 in New Orleans. The MOMENTUM 3 study compared the HeartMate 3 Left Ventricular Assist System (LVAS) to the HeartMate II LVAS in treating advanced stage heart failure, and is the largest LVAD trial in the world to evaluate both short-term and long-term patients in a single study. The study results demonstrated patients receiving the HeartMate 3 LVAS had an 86.2 percent survival rate with freedom from disabling stroke and reoperation to repair or replace the device.
Corsens Medical Ltd. announced that it has received clearance for a Pre-Marketing Notification (510(k)) with the U.S. Food and Drug Administration (FDA) for its Corsens Cardiac Monitor (patents pending).
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Shimadzu's latest generation interventional lab angiography imaging system, the Trinias, enables advanced imaging ...
December 7, 2016 — Boston Scientific announced a definitive agreement to acquire certain manufacturing assets and ...

December 7, 2016 — Abbott has filed a complaint to terminate its proposed acquisition of Alere based on the substantial ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
December 7, 2016 — Abiomed Inc. announced it has expanded its U.S. Food and Drug Administration (FDA) pre-market ...
December 7, 2016 — Teleflex Inc. and Vascular Solutions Inc. announced that the companies have entered into a definitive ...
December 7, 2016 — At the 102nd Scientific Assembly and Annual Meeting of the Radiological Society of North America ...

 December 19, 2016
December 19, 2016