Jan. 27, 2026 — BURL Concepts, a private company developing a rapid, automated and affordable test for evaluating ...
Jan. 27, 2026 — Cytokinetics, Inc. has announced that Myqorzo (aficamten) is now available for prescription in 5 mg, 10 ...
Jan. 27, 2026 — A new national study reveals a stark disconnect between Americans’ desire for preventive cardiac ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Jan. 27, 2026 — Robocath has launched the world’s first FIH (First-In-Human) clinical study evaluating its new robotic ...
Jan. 26, 2026 — AISAP, a provider of AI point-of-care diagnostics, has published a new clinical study in the peer ...
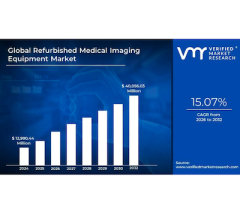
Jan. 11, 2026 — The Global Refurbished Medical Imaging Equipment Market Size is projected to grow at a CAGR of 15.07% ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Jan. 20, 2026 — Abbott has received CE Mark in Europe for the TactiFlex Duo Ablation Catheter, Sensor Enabled to treat ...
Jan. 20, 2026 — Kardium Inc. has announced the publication of the PULSAR clinical trial results in the Journal of the ...
Jan. 20, 2026 — Polarean, a commercial-stage medical imaging company advancing functional MRI of the lungs, has expanded ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
On Feb. 19, at 7 p.m. eastern time, Cleerly will present a live webinar on “Women's Heart Health: Uncovering Hidden ...
Jan. 15, 2026 — Boston Scientific Corp. and Penumbra, Inc. have entered into a definitive agreement under which Boston ...
Jan. 16, 2026 — MemorialCare Heart & Vascular Institute has launched the FDA-approved Symplicity Renal Denervation (RDN) ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
Jan. 6, 2026 — UltraSight, a provider of AI-guided cardiac imaging workflows, has announced FDA clearance to expand its ...
Jan. 13, 2026 – Innovative Health, Inc. has received its 50th clearance from FDA to reprocess single-use medical devices ...
Jan. 13, 2026 — A streamlined, nurse-led response for hospitalized patients experiencing an acute stroke at a Texas ...


 January 28, 2026
January 28, 2026