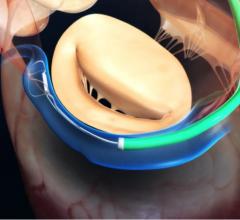
Startup cardiovascular device manufacturer Valtech Cardio Ltd. announced it has received CE Marking for its Cardioband mitral reconstruction system (Cardioband), a proprietary, implantable mitral reconstruction device with a transfemoral, transseptal delivery system for mitral valve repair.
Vital Images Inc. recently introduced the VioSuite Image Management and Vitality Solutions Business Intelligence product families, providing healthcare administrators with a robust set of tools to improve system-wide image access and image data analytics. VioSuite and Vitality Solutions, as well as the company’s flagship products VitreaAdvanced and VitreaView software, were showcased at the 9th annual Health Information and Management Systems Society (HIMSS) Asia Pacific Conference and Exhibition.
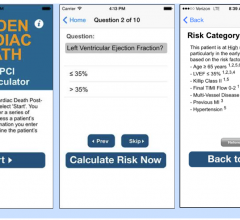
September 14, 2015 — Healthcare providers can assess a patient’s risk of sudden cardiac death (SCD) following a ...
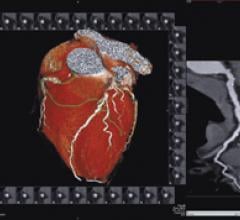
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
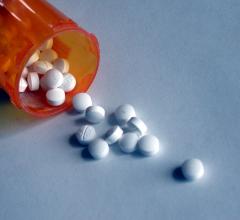
New Haven Pharmaceuticals Inc. announced U.S. Food and Drug Administration (FDA) approval of Durlaza (aspirin) 24-hour extended-release capsules for the secondary prevention of stroke and acute cardiac events, including myocardial infarction.
Zevacor Molecular (Zevacor), manufacturer and distributor of positron emission tomography (PET) and single photon emission computed tomography (SPECT) radiopharmaceuticals, announced the arrival of a 70 MeV Cyclotron at its new production facility in Noblesville, Indiana.
Westinghouse Electric Company and NorthStar Medical Radioisotopes announced a memorandum of understanding to explore producing medical radioisotopes from the core of commercial nuclear reactors, and methods of global distribution. The exploration involves generating the most widely used radioisotope in medical diagnostic imaging by treating an isotope of the chemical element molybdenum rather than enriched uranium.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
September, 11, 2015 – Japanese medical vendor Nipro Corp. signed a definitive agreement to acquire Infraredx Inc., maker ...

The cardiovascular service line, whether existing within the confines of an acute-care environment or outpatient setting ...

For any cardiology department looking to upgrade or replace its cardiovascular information system (CVIS), the main ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

Two key requirements for today’s cardiovascular information systems (CVIS) are solid integrations with both enterprise ...
Sunshine Heart Inc. announced commencement of a new study examining the impact of the C-Pulse system on pulmonary circulation and right heart related to pulmonary hypertension and heart failure.
AliveCor Inc. announced significant milestones related to patients with atrial fibrillation (AFib) using the AliveCor Mobile ECG. Since receiving the first U.S. Food and Drug Administration (FDA) clearance for the AF Detector, an algorithm to detect atrial fibrillation in an ECG (electrocardiogram), 30 percent of AliveCor patients have received an AFib detection.
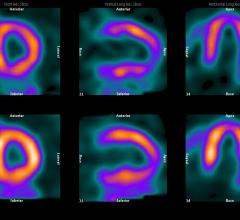
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The Carillon mitral contour system is projected to be a cost-effective treatment option when compared to a typical regimen of optimal medical treatment (OMT), the present standard of care for functional mitral regurgitation (FMR).
Researchers at Thomas Jefferson University showed that a simple questionnaire, evaluation and pulse-oximetry monitoring can lead to early detection of sleep apnea in patients hospitalized for congestive heart failure (CHF).
St. Jude Medical Inc. announced that five-year results from the FAME trial have confirmed the long-term benefits of fractional flow reserve (FFR) in guiding percutaneous coronary intervention (PCI) over angiography alone.

 September 14, 2015
September 14, 2015