May 6, 2016 — Atrial fibrillation patients taking warfarin are at higher risk of developing kidney failure if ...
Atrial Fibrillation
This channel includes news and new technology innovations for the treatment of atrial fibrillation, also referred to as AF or afib. AF is a cardiac arrhythmia caused by irregular and often rapid heart rate. It is caused by the upper chambers (the atria) beating irregularly and uncoordinated with the lower ventricle chambers of of the heart. Symptoms include weakness with heart palpitations and shortness of breath. The conditional can lead to an increased risk of stroke and heart failure. AF episodes can cause the blood in the atria to stagnate and form clots, usually within the left atrial appendage (LAA). The clots can flow to the brain and cause a stroke. Treatments include anticoagulation therapy to dissolve clots, catheter or surgical ablation and LAA occlusion.
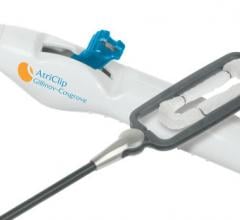
April 29, 2016 — AtriCure Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the AtriClip PRO2 ...
April 20, 2016 — In the first large randomized trial to directly compare two approaches to preventing atrial ...
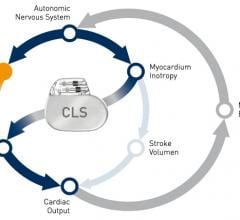
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
David Holmes, M.D., professor of medicine, Mayo Clinic College of Medicine and consultant, Department of Internal ...
April 8, 2016 — New data from an ongoing post-marketing study confirm the safety profile of Xarelto (rivaroxaban) was ...
AliveCor, Inc., the leader in FDA-cleared electrocardiogram (ECG) technology for mobile devices, announced today the ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
March 17, 2016 — Nearly half of all atrial fibrillation (AF) patients at the highest risk for stroke are not being ...
March 16, 2016 — The Valley Hospital in Ridgewood, N.J., is one of 15 U.S. sites currently enrolling patients in a ...
March 14, 2016 — Biotronik announced that its BioMonitor 2 heart monitor is now available for patients in the United ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
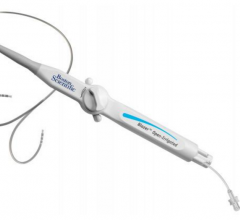
March 11, 2016 — Boston Scientific has received U.S. Food and Drug Administration (FDA) approval for the Blazer Open ...
February 18, 2016 — AtriCure Inc. announced that the first patient was enrolled and treated at PinnacleHealth Hospitals ...
January 6, 2016 — A new study determined that the Watchman left atrial appendage closure device is more cost-effective ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
December 22, 2015 — In 2015, results from the ITALIC and CvLPRIT trials, along with studies examining lifestyle factors ...
December 17, 2015 — The ROCKET AF Clinical Trial Executive Committee announced its secondary analysis of the phase III ...
December 8, 2015 — Biotronik announced the first patient enrollments to the B3 clinical trial. The study will evaluate ...

 May 06, 2016
May 06, 2016