February 22, 2017 — If hospitals can perform more transradial, same-day percutaneous coronary intervention (PCI) ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
February 17, 2017 — Medtronic plc announced that its coronary portfolio will now include the DxTerity Diagnostic ...
The supplies you use in your cath lab are complex and very valuable. Protecting your investment and uncovering new ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
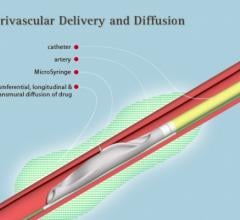
February 15, 2017 — Mercator MedSystems announced that the national co-principal investigators of the company’s DANCE tr ...
February 15, 2017 — Better hospital supply chain management leads to better quality of care and supports patient safety ...
February 15, 2017 — Doctors at the University of Alabama at Birmingham have implemented the first U.S. Food and Drug ...
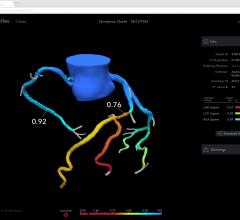
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
February 15, 2017 — The PRO-Kinetic Energy Cobalt Chromium (CoCr) Coronary Stent System from Biotronik has gained U.S ...
In the current era of healthcare reform and the push toward evidence-based medicine to both lower costs and improve ...
February 14, 2017 — The National Institute for Health and Care Excellence (NICE) in the United Kingdom recently issued g ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
Stents were the key focus of interventional cardiology for more than 20 years, but the focus has changed in recent years ...
February 13, 2017 — Vascular Dynamics Inc. (VDI) announced that the U.S. Food and Drug Administration (FDA) has approved ...

In the past few years there have been a number of device therapies developed to treat heart failure (HF). This is partly ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
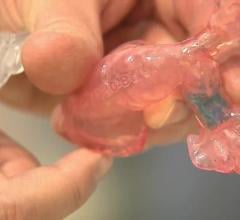
When a pediatric patient at Children’s Hospital Los Angeles needed a custom-build stent to repair his pulmonary artery ...
February 9, 2017 — Janssen Research & Development LLC (Janssen) announced that the Phase 3 COMPASS trial is stopping ...
February 8, 2017 — Medtronic plc announced receipt of an investigational device exemption (IDE) from the U.S. Food and ...

 February 22, 2017
February 22, 2017