Troponins are a family of proteins found in skeletal and heart (cardiac) muscle fibers that produce muscular contraction ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

Reflecting a trend toward the increased use of computed tomography (CT) in cardiology, Siemens Healthineers launched a ...
March 21, 2019 — Clinicians should use echocardiography when determining whether patients with heart failure and a ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
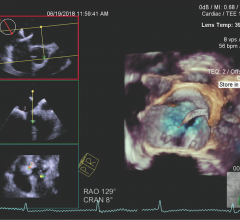
March 20, 2019 — At the American College of Cardiology’s (ACC) annual meeting, March 16-18 in New Orleans, Philips ...
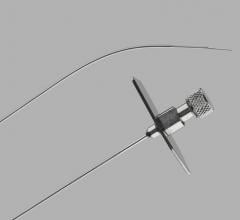
March 20, 2019 — Cook Medical is recalling one lot of its Transseptal Needle due to a manufacturing error that resulted ...
The American College of Cardiology (ACC) released a list of the latest practice-changing presentations at the ACC.19 ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
March 19, 2019 — Late-breaking results confirm the HeartFlow FFRct (fractional flow reserve computed tomography) ...

As many as one in four patients who undergo cath lab interventions can benefit from a technology that identifies the ...
March 14, 2019 — Beginning in mid-2019, hospitals performing transcatheter valve repair and replacement will be able to ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
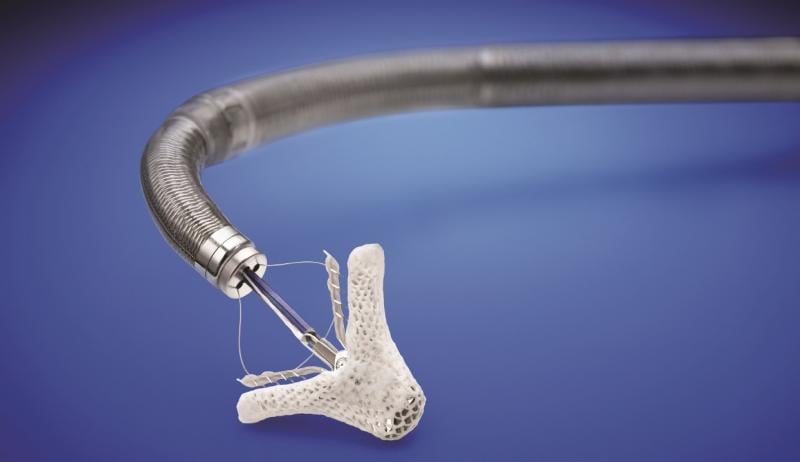
March 14, 2019 — The U.S. Food and Drug Administration (FDA) approved the MitraClip heart valve repair device for ...
March 12, 2019 — V-Wave Ltd. announced that renowned heart failure cardiologist William T. Abraham, M.D., is joining V ...
Can computed tomography (CT) angiography accurately measure fractional flow reserve (FFR)? Even if it can, is CT-derived ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
March 7, 2019 — Rising incidence of congenital heart disease and increasing awareness of transcatheter pulmonary valve ...
March 6, 2019 – Swiss-based M.A. MedAlliance SA has been granted Breakthrough Device Designation from the U.S. Food and ...
March 5, 2019 – The U.S. Food and Drug Administration (FDA) cleared Abbott's Resting Full-cycle Ratio (RFR) ...


 March 22, 2019
March 22, 2019