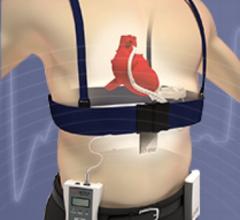
February 13, 2019 — Le Bonheur Children's Hospital cardiologists in Memphis, Tenn., implanted the Amplatzer Piccolo ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
William O'Neill, M.D., highlights best practice protocols based on Impella Quality database and real-world evidence ...
February 12, 2019 — A study presented at the 2018 annual meeting of the Cardiovascular and Interventional Radiology ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
February 11, 2019 — Heart failure experts at the National Research Center for Cardiac Surgery in Astana, Kazakhstan ...
This is an example of an arterial venous malformation (AVM) in the brain imaged on a Canon Alphenix Alpha angiography ...
February 7, 2019 — Medtronic plc recently received U.S. Food and Drug Administration (FDA) approval for the Valiant ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
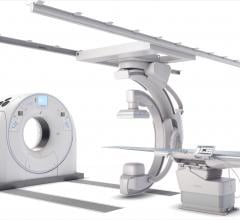
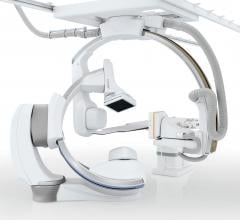
February 6, 2019 — Canon Medical Systems USA Inc. recently introduced a new angiography configuration featuring its ...
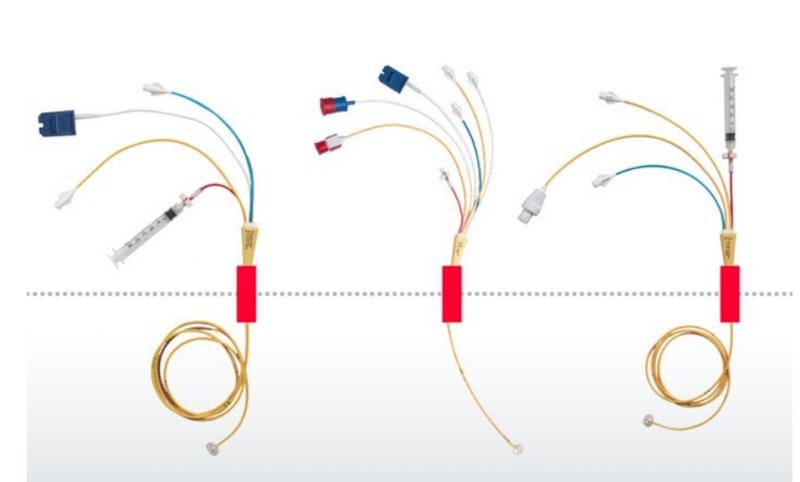
Edwards Lifesciences is recalling its 131F7, 131F7J, 131F7P, 131VF7P, 151F7 Swan-Ganz Thermodilution Catheters ...
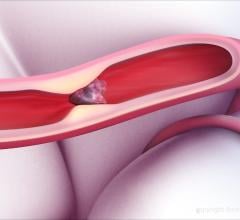
February 5, 2019 — Stroke survivors have better quality of life three months after their stroke if the clot that caused ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
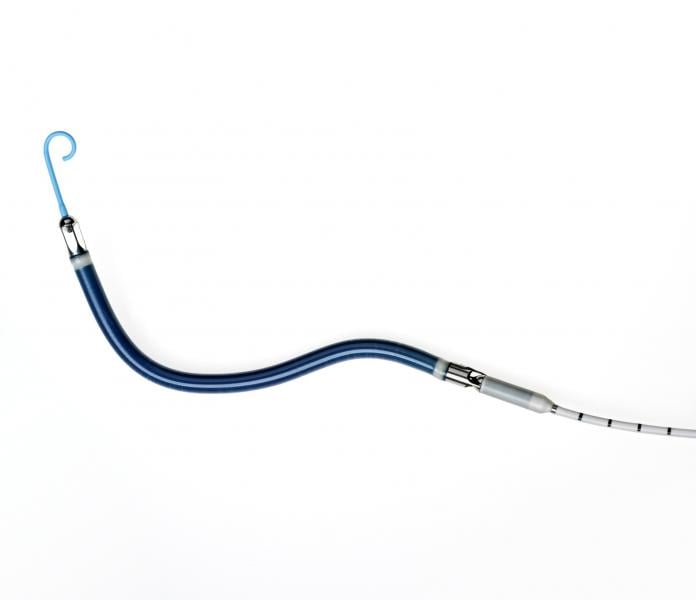
The U.S. Food and Drug Administration (FDA) sent a letter to cardiologists this week to explain its evaluation of high ...
February 4, 2019 — Canon Medical Systems USA recently introduced its next generation of interventional systems – the ...

February 1, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) m ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
February 1, 2019 — Merit Medical Systems Inc. announced that the PreludeSync Distal Compression Device is now available ...
January 31, 2019 — Cardiovascular Systems Inc. announced that the first patients in the United States were treated using ...

The higher expense and lower frame rates of 3-D cardiac ultrasound systems have limited their adoption over the past ...


 February 13, 2019
February 13, 2019