September 30, 2019 – Data from the EVOLVE Short DAPT study found that shortened three-month dual antiplatelet therapy ...
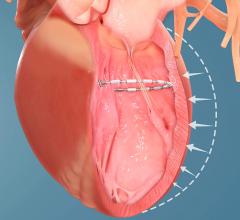
September 27, 2019 — Ancora Heart Inc. announced results from an interim analysis of heart failure patients treated in ...
September 27, 2019 — Abiomed’s newest heart pump, the Impella 5.5 with SmartAssist, has received U.S. Food and Drug ...
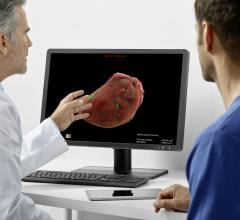
September 27, 2019 — EchoPixel introduced what it calls the first-ever intraoperative software to provide naked-eye ...
September 26, 2019 — New data from the Phase IV independent TWILIGHT trial showed Brilinta (ticagrelor) monotherapy redu ...
September 26, 2019 — The U.S. Food and Drug Administration (FDA) has cleared three modules of AI-Rad Companion Chest CT ...
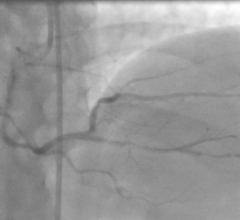
September 25, 2019 — Ten-year survival rates are similar for bypass surgery and coronary stenting with drug-eluting ...
September 24, 2019 – Medtronic announced U.S. Food and Drug Administration (FDA) approval and U.S. launch of the Evolut ...
There was a 77 percent increase in survival in cardiogenic shock patients treated using a new protocol in the National ...
September 20, 2019 — BioTrace Medical Inc. announced the company’s key activities at the 31st annual Transcatheter ...
September 20, 2019 — Low-dose aspirin does not prolong disability-free survival of healthy people over 70, even in those ...
September 18, 2019 — Early use of an implantable cardioverter-defibrillator (ICD) after primary coronary intervention ...
September 18, 2019 — Discharge of patients with suspected acute coronary syndromes under a 0- and 1-hour high ...
September 18, 2019 — AstraZeneca announced the U.S. Food and Drug Administration (FDA) has granted Fast Track ...
September 17, 2019 — The European Society of Cardiology (ESC) published new guidelines on the diagnosis and management ...

 September 30, 2019
September 30, 2019