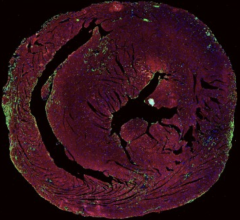
November 3, 2022 — In the past several years, myocarditis has been of public interest because of cases associated with ...
Coronavirus (COVID-19)
This page contains medical information for clinicians on the 2019 Novel Coronavirus (COVID-19, also called 2019-nCoV, and now clinically SARS‐CoV‐2). This section includes articles that pertain to clinicians and cardiologists on the virus, new technologies being deployed to fight the virus and clinical information from various sources. Here are direct links for medical professionals to COVID-19 resources from the U.S. Food and Drug Administration (FDA), Centers for Disease Control (CDC) and the World Health Organization (WHO). Daily world-wide statistics on the coronavirus outbreak are available from the WHO Situations Reports. Here is the Centers for Medicare and Medicaid Services (CMS) frequently asked questions and answers (FAQs) for healthcare providers regarding Medicare payment for laboratory tests and other services related to the COVID-19.
November 2, 2022 — A comprehensive review and meta-analysis of published research confirm that young adults (40 years ...

Here is what you and your colleagues found to be most interesting in the fields of diagnostic and interventional ...
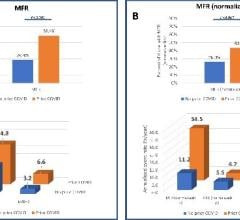
During the COVID-19 pandemic, many cardiology departments were faced with the daunting task of supporting inpatient and ...
October 27, 2022 — New data analysis from the Smidt Heart Institute at Cedars-Sinai found that deaths from heart ...
October 17, 2022 — A national study suggests that risk factors for cardiovascular disease, such as age, smoking and ...
September 26, 2022 — Kawasaki disease (KD) is the leading cause of acquired heart disease in children in developed ...
COVID-19 has posed challenges for physicians whose cardiac patients are at-risk and reluctant to schedule an office ...
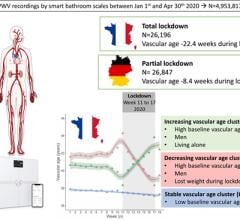
September 7, 2022 — A new paper in European Heart Journal - Digital Health, published by Oxford University Press ...
August 26, 2022 — COVID mRNA vaccines are associated with a decreased risk of death in patients with heart failure ...
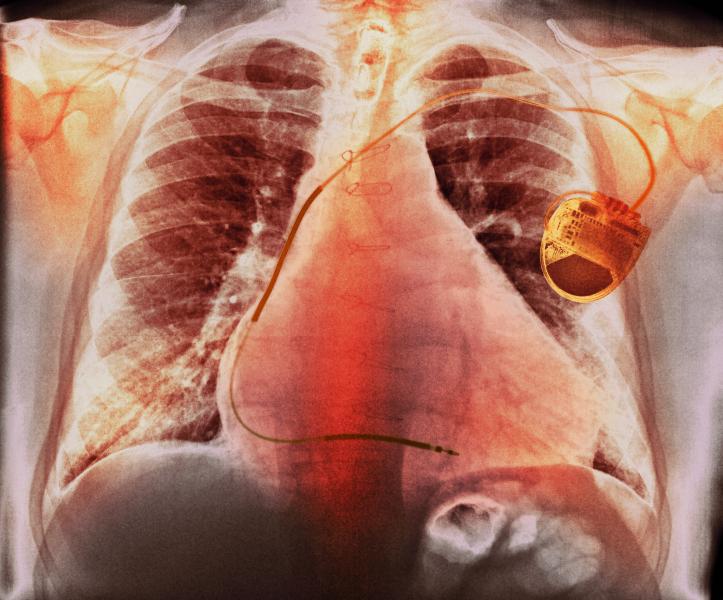
Arrhythmias can be described as irregular heart rhythms that are brought on by numerous factors, including heart damage ...
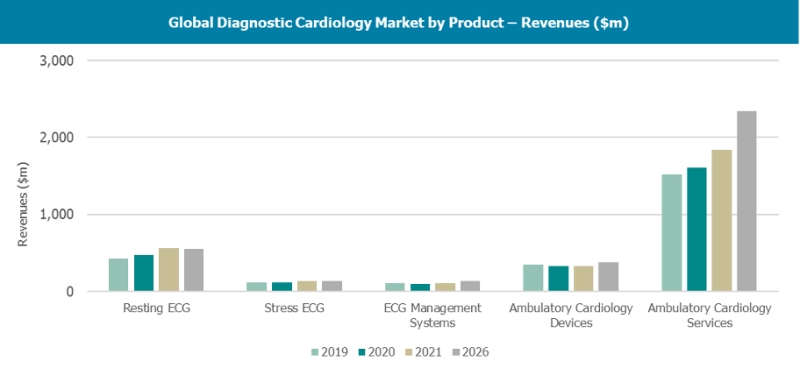
Like most healthcare markets, the diagnostic cardiology market has had a bumpy ride in recent years. The COVID-19 pandem ...
August 23, 2022 — In a detailed analysis of nearly 43 million people, ages 13 and older, who received at least one dose ...

Hospitals in the U.S. are suffering under a debilitating post-pandemic financial and operational trauma. Ascension poste ...

Anthony S. Fauci, MD, who was instrumental in leading the country through the COVID-19 pandemic, announced today through ...
August 19, 2022 — Patients with prior COVID may be twice as likely to have unhealthy endothelial cells that line the ...

This photo gallery is designed to show the impact of the novel coronavirus (COVID-19, SARS-CoV-2) on both healthcare and ...

 November 03, 2022
November 03, 2022