July 27, 2022 — Heart damage is common among patients hospitalized with COVID-19, leading many to wonder how the virus ...
Coronavirus (COVID-19)
This page contains medical information for clinicians on the 2019 Novel Coronavirus (COVID-19, also called 2019-nCoV, and now clinically SARS‐CoV‐2). This section includes articles that pertain to clinicians and cardiologists on the virus, new technologies being deployed to fight the virus and clinical information from various sources. Here are direct links for medical professionals to COVID-19 resources from the U.S. Food and Drug Administration (FDA), Centers for Disease Control (CDC) and the World Health Organization (WHO). Daily world-wide statistics on the coronavirus outbreak are available from the WHO Situations Reports. Here is the Centers for Medicare and Medicaid Services (CMS) frequently asked questions and answers (FAQs) for healthcare providers regarding Medicare payment for laboratory tests and other services related to the COVID-19.
Organizers of the European Society of Cardiology (ESC) Congress have released plans and announced 10 Hot Line ...
July 11, 2022 — Smoking traditional or non-combustible cigarettes while wearing a surgical mask results in a two-fold ...
During the COVID-19 pandemic, many cardiology departments were faced with the daunting task of supporting inpatient and ...
July 7, 2022 — Telemedicine has expanded rapidly since the beginning of the pandemic and is being increasingly adopted ...
July 5, 2022 — The International Journal of Cardiology published an article on the findings of how the Coronavirus ...
COVID-19 has posed challenges for physicians whose cardiac patients are at-risk and reluctant to schedule an office ...
June 27, 2022 — Three articles and an accompanying editorial provide information on the effects of Long COVID in the Deu ...
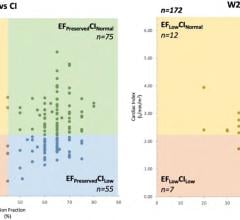
June 24, 2022 — Henry Ford Health was part of a multi-institutional heart failure study that was launched and executed ...
June 23, 2022 — The COVID-19 vaccination is not associated with an increased risk of heart attack or stroke in patients ...
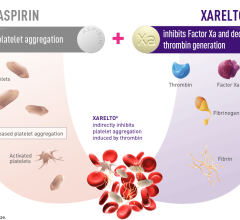
May 31, 2022 — Findings from the XARELTO (rivaroxaban) Phase 3 COMPASS Long-Term Open Label Extension (LTOLE) study and ...
May 23, 2022 — New findings from the Ascension Health System’s internal National Cardiovascular Data Registry (NCDR) ...
May 19, 2022 — The latest analysis from The North American COVID-19 STEMI (NACMI) was presented today as late-breaking ...
May 17, 2022 — Results from a new study show success of intended same day discharge (SDD) improves over time for ...
May 16, 2022 — A new report by a team of cardiologists from the Heart & Vascular Hospital, Hackensack Meridian ...
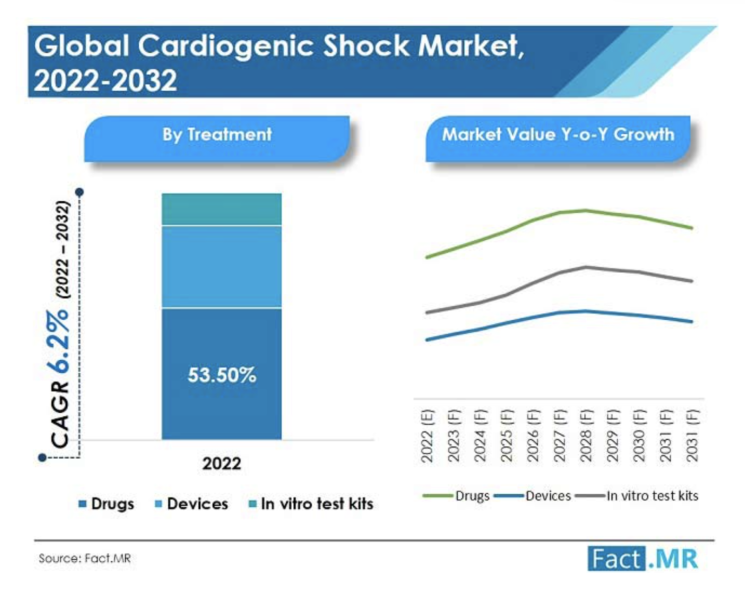
The global cardiogenic shock market was valued at US$ 3.29 Bn in 2021, and is expected to reach US$ 6.32 Bn by 2032 ...

 July 27, 2022
July 27, 2022