Robert Kowal, M.D., chief medical officer of the Medtronic cardiac rhythm and heart failure division, said there has ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
December 21, 2020 — U.S. Food and Drug Administration (FDA) approved updated labeling December 17 for Abbott's HeartMate ...
December 16, 2020 - Intelerad Medical Systems, a provider of enterprise imaging workflow solutions, has acquired ...
December 14, 2020 - Foldax Inc. today announced that the U.S. Food and Drug Administration (FDA) has granted an ...
December 9, 2020 — SoniVie, an Israeli company developing the Therapeutic Intra-Vascular Ultrasound (TIVUS) System to ...

View the November-December 2020 digital edition of Diagnostic and Interventional Cardiology (DAIC) magazine, include ...
December 2, 2020 — A 2-month-old infant diagnosed with COVID-19 (SARS-CoV-2) experienced reversible myocardial injury ...
December 2, 2020 — The Centers for Medicare and Medicaid Services (CMS) finalized updates to Medicare coverage policies ...
December 1, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
December 1, 2020 — A recent publication demonstrated procedural efficiency for MitraClip transcatheter mitral valve ...
November 18, 2020 — A late-breaking study at the 2020 American Heart Association (AHA) Scientific Sessions showed nearly ...
November 18, 2020 — The primary results from the RIVER Trial, Rivaroxaban for Valvular Heart disease and Atrial ...

November 18, 2020 -- A new American Heart Association (AHA) collaborative model for COVID-19 (SARS-CoV-2) research ...
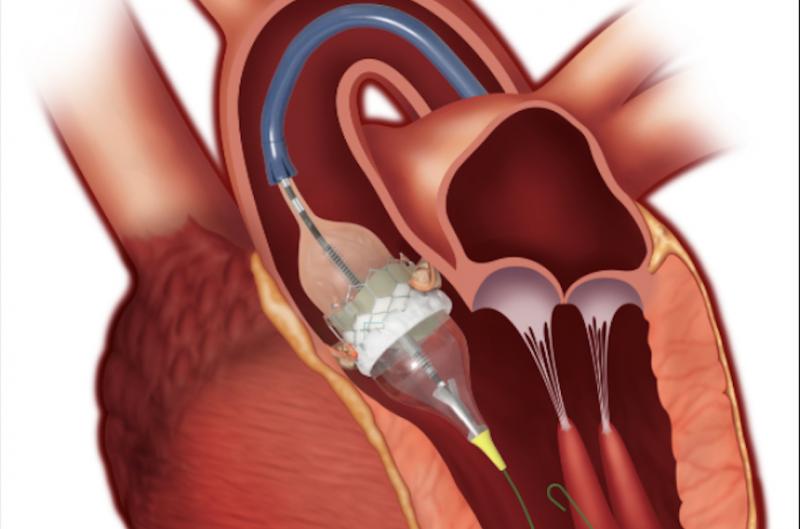
November 17, 2020 — Since the approval of the first transcatheter aortic valve replacement (TAVR) device in 2011, more ...

November 17, 2020 — Boston Scientific Corp. announced today it is immediately retiring the entire Lotus Edge ...


 December 22, 2020
December 22, 2020