November 13, 2020 — The new U.S. Food and Drug Administration (FDA) cleared XO Score system was successfully used to ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
November 12, 2020 — Abiomed recently announced new PROTECT III study data that demonstrates reduced rates of MACCE ...
Keith Ellis, M.D., is the director of cardiovascular services and the director of the Chest Pain Center at Houston ...
November 2, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Here are some of the key takeaways from the late-breaking interventional cardiology and structural heart trials ...
Oct. 29, 2020 — Boston Scientific initiated the CHAMPION-AF clinical trial to evaluate the safety and efficacy of the ...
The September-October 2020 digital edition of Diagnostic and Interventional Cardiology (DAIC) magazine include links to ...
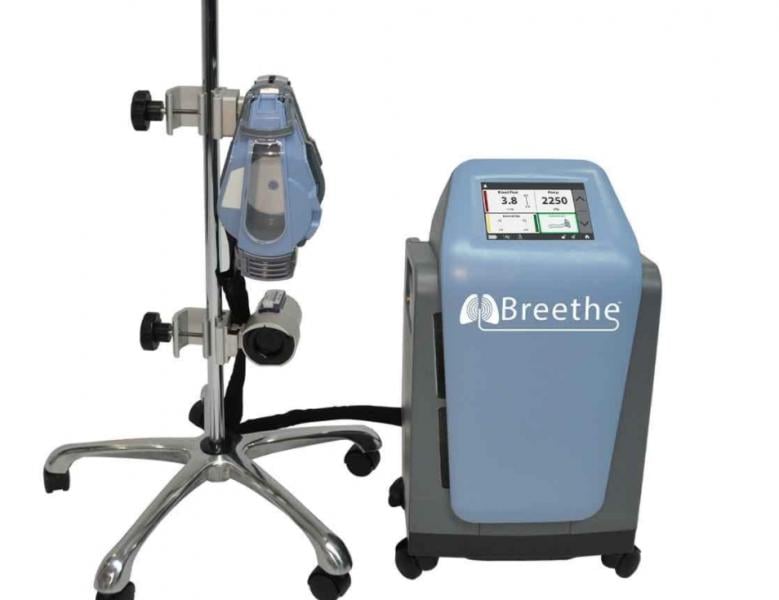
October 26, 2020 — The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to Abiomed for an all-in-one ...
October 26, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the Medtronic Abre venous self-expanding ...
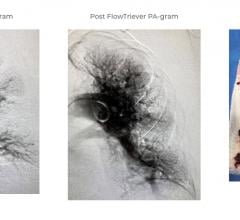
October 23, 2020 — Positive results were reported on the first 230 patients enrolled in its FLASH study, a real world p ...
October 22, 2020 – In the FORECAST randomized clinical trial, the use of fractional flow reserve (FFR) derived from ...
Chuck Simonton, M.D., chief medical officer at Abiomed, explains when advanced hemodynamic support is required in COVID ...
Chuck Simonton, M.D., chief medical officer at Abiomed, discusses some of the new technologies and clinical trials the ...
David Cohen, M.D., presents late-breaking data from the STS/ACC Transcatheter Valve Registry (TVT) showing the impact of ...
Doctor Hans-Josef Feistritzerm, Heart Center of Leipzig, Germany, presents data on the use of general vs. local ...


 November 13, 2020
November 13, 2020