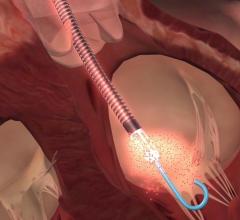
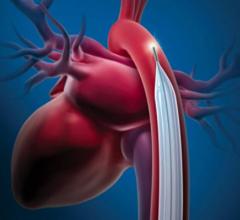
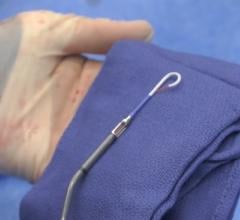
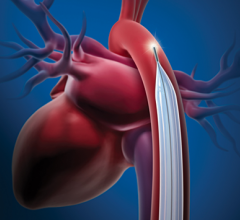
December 28 2022 — Abiomed announces the United States Food and Drug Administration (FDA) has approved the version of Im ...
Intra-Aortic Balloon Pumps (IABP)
This channel includes news and new technology innovations for intra-aortic balloon pumps (IABP). IABPs are a type of hemodynamic support device that is inserted into the aorta to help augment the flow of blood with a pulsing balloon that pushes the blood volume out of the the aorta to help perfuse organs and tissues.

The U.S. Food and Drug Administration (FDA) has issued a Class I recall on the Arrow AutoCAT 2 and AC3 Intra-Aortic ...
October 26, 2022 — Abiomed (Nasdaq: ABMD) announces a new program to address healthcare disparities in underserved ...
December 16, 2021 — Datascope/Getinge/Maquet has recalled the Cardiosave Hybrid and Cardiosave Rescue Intra-Aortic ...
Srihari Naidu, M.D., FACC, FAHA, FSCAI, is the director of both the cardiac cath labs and Hypertrophic Cardiomyopathy ...
November 2, 2021 — Datascope/Getinge/Maquet is recalling its Cardiosave Hybrid/Rescue Intra-Aortic Balloon Pumps (IABP) ...
Navin Kapur, M.D., FAHA, FACC, FSCAI, director, Acute Mechanical Circulatory Support Program and executive director of ...
November 19, 2019 — Since Maquet/Datascope first recalled all of its intra-aortic balloon pumps (IABP) due to reports ...
Jeffrey J. Popma, M.D., director of interventional cardiology clinical services at Beth Israel Deaconess Medical Center ...
A discussion with William O'Neill, M.D., director of the structural heart program, Henry Ford Hospital, and Michele ...
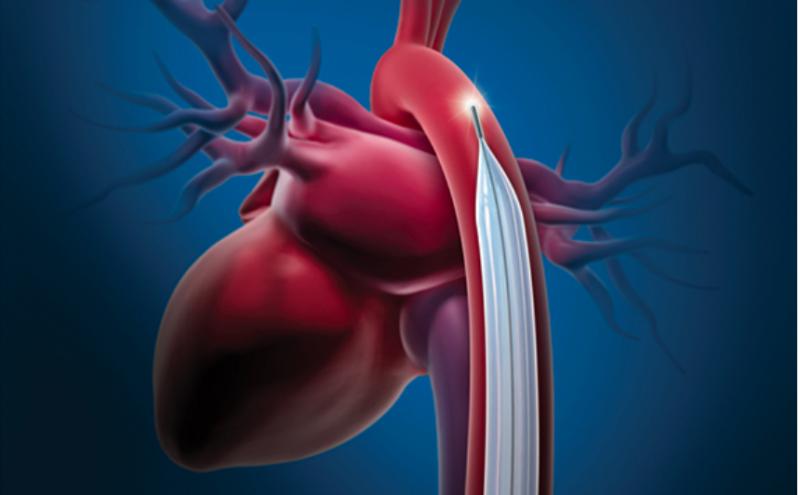
July 24, 2019 — The U.S. Food and Drug Administration announced Maquet/Datascope is recalling all intra-aortic balloon ...
This podcast is a discussion with William O'Neill, M.D., director of the structural heart program, Henry Ford Hospital ...
November 5, 2018 — The U.S. Food and Drug Administration (FDA) is evaluating recent reports of Getinge's Maquet ...
September 24, 2018 — Getinge is voluntarily initiating a worldwide recall involving a field correction of approximately ...
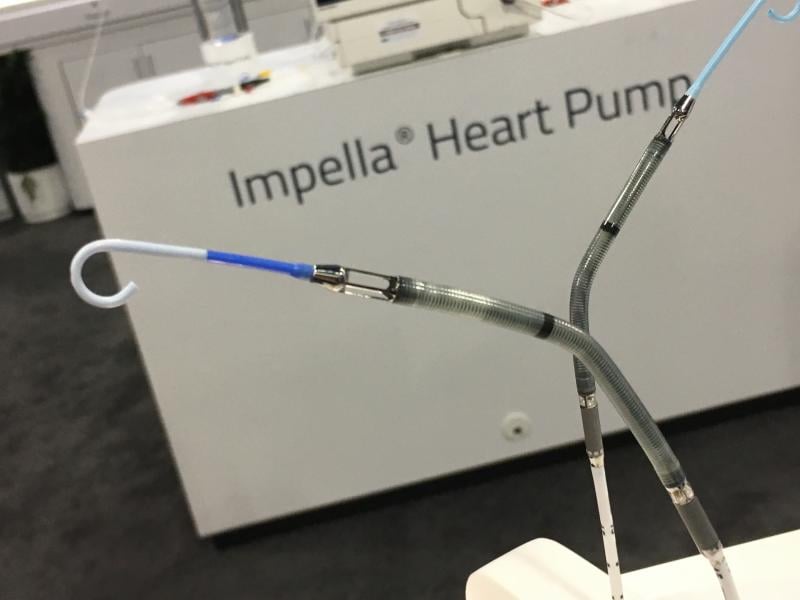

 December 28, 2022
December 28, 2022